Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.tet.2017.09.017 Martina Drábiková , Soňa Krajčovičová , Miroslav Soural
|
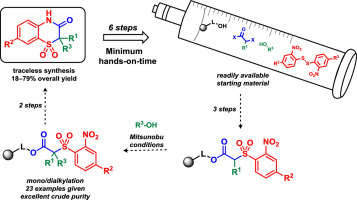
Herein, we report the first examples of the Mitsunobu alkylation of β-alkoxycarbonyl 2-nitrobenzenesulfones. Wang resin was acylated with α-halocarboxylic acids followed by the reaction with 2-nitrothiophenols. After oxidation with m-chloroperbenzoic acid, the immobilized β-alkoxycarbonyl 2-nitrobenzensulfones were subjected to alkylation with various alcohols. The reaction outcome strongly depended on the selection of the alkylating species. After the reduction of the nitro group, acid-mediated cleavage and subsequent cyclization, the C2-(di)substituted benzothiazin-3(4H)-one 1,1-dioxides were obtained in high crude purities and good overall yields.
中文翻译:

β-烷氧基羰基2-硝基苯砜的Mitsunobu C-烷基化及其在快速合成新型苯并噻嗪衍生物中的用途
在此,我们报道了β-烷氧基羰基2-硝基苯砜的Mitsunobu烷基化的第一个例子。Wang树脂用α-卤代羧酸酰化,然后与2-硝基硫代苯酚反应。与氧化后米氯过苯甲酸,固定化β -烷氧基羰2- nitrobenzensulfones进行烷基化与各种醇。反应结果在很大程度上取决于烷基化物质的选择。在硝基还原,酸介导的裂解和随后的环化之后,以高的粗纯度和良好的总收率获得了C 2-(二)取代的苯并噻嗪-3(4 H)-1,1-二氧化物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号