Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.tet.2017.09.021 Kamil Lisiecki , Krzysztof K. Krawczyk , Piotr Roszkowski , Jan K. Maurin , Armand Budzianowski , Zbigniew Czarnocki
|
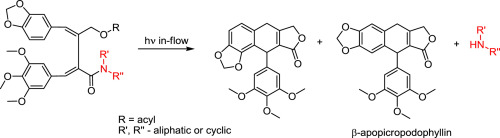
During the attempted photochemical cyclization of 2,3-bisbenzylidene-γ-hydroxybutyric acid cyclic amide ester, it was observed that a γ-butyrolactone ring was formed, which was concurrent with the release of the amine fragment from the amide. The process occurs with high yield giving rise to the formation of β-apopicropodophyllin and its regioisomer. Additional experiments confirmed the photochemical nature of this transformation, and that it is independent from the photocyclization of the benzylidene groups – a typical reactivity for the members of the fulgide family. In contrast to the latter UV-driven cyclization, the photochemical cleavage of the amide was proven to proceed under irradiation with visible light.
中文翻译:

与鬼臼毒素有关的环木脂素合成过程中叔酰胺的不寻常可见光光解裂解
在尝试对2,3-双亚苄基-γ-羟基丁酸环状酰胺酯进行光化学环化的过程中,观察到形成了γ-丁内酯环,这与胺片段从酰胺中的释放同时发生。该过程以高收率发生,导致β-载鬼臼苦素及其区域异构体的形成。额外的实验证实了这种转变的光化学性质,并且它独立于亚苄基的光环化作用-这是the灭肽家族成员的典型反应活性。与后者的紫外线驱动环化相反,已证明酰胺的光化学裂解是在可见光照射下进行的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号