Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.tetlet.2017.09.038 Yuriy I. Horak , Roman Z. Lytvyn , Yevhen-Oleh V. Laba , Yuriy V. Homza , Vladimir P. Zaytsev , Maryana A. Nadirova , Tatiana V. Nikanorova , Fedor I. Zubkov , Alexey V. Varlamov , Mykola D. Obushak
|
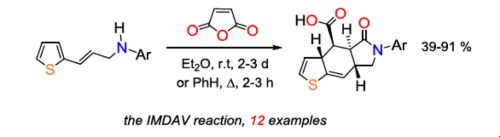
The reaction of readily accessible 3-(thien-2-yl)allylamines with maleic anhydride, followed by a domino sequence involving successive acylation/[4+2] cycloaddition steps, leads to the formation of the thieno[2,3-f]isoindole core. The key step, the intramolecular Diels-Alder vinylaren (IMDAV) reaction, proceeds with high level of diastereoselectivity and with formation of a single diastereoisomer of the target product 4,4a,5,6,7,7a-hexahydro-3aH-thieno[2,3-f]isoindole-4-carboxylic acids in excellent yields. If the reaction is carried out at room temperature, it occurs in 2–3 days and the proton migration (H-shift) does not take place at the last stage. In boiling benzene, the reaction is complete after three hours, but in this case a slight impurity of byproducts bearing aromatic thiophene ring – 4a,5,6,7,7a,8-hexahydro-4H-thieno[2,3-f]isoindole-4-carboxylic acids is formed.
中文翻译:

分子内Diels-Alder乙烯基噻吩(IMDAV)反应:硫代[2,3 - f ]异吲哚-4-羧酸的简便方法
易于获得的3-(thien-2-yl)烯丙胺与顺丁烯二酸酐的反应,然后是涉及连续酰化/ [4 + 2]环加成步骤的多米诺序列,导致噻吩并[2,3- f ]的形成异吲哚核。的关键步骤中,分子内狄尔斯-阿尔德vinylaren(IMDAV)反应,具有高非对映选择性水平的,并与形成所述目标产物甲基-4,4a,5,6,7,7a-六氢3a的单个非对映体的前进ħ -噻吩并[2,3 - f ]异吲哚-4-羧酸的收率很高。如果反应在室温下进行,则反应会在2到3天之内发生,并且质子迁移(H-shift)不会在最后一个阶段发生。在沸腾的苯中,反应在三个小时后完成,但在这种情况下,带有芳族噻吩环的副产物中有少量杂质– 4a,5,6,7,7a,8-六氢-4 H-硫代[2,3- f形成]异吲哚-4-羧酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号