当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric synthetic approach to a functionalized azabicyclo[3.3.1]nonane moiety of (+)-sarain A
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.tetlet.2017.09.045 Jiao Meng , Yu Wang , Yong Qin , Xiao-Yu Liu
中文翻译:

(+)-sarain A的功能化氮杂双环[3.3.1]壬烷部分的不对称合成方法
更新日期:2017-09-18
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-18 , DOI: 10.1016/j.tetlet.2017.09.045 Jiao Meng , Yu Wang , Yong Qin , Xiao-Yu Liu
|
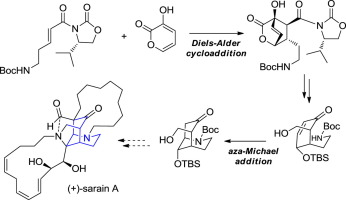
This paper describes our efforts toward the asymmetric synthesis of sarain A which led to efficient preparation of a functionalized azabicyclo[3.3.1]nonane moiety. The key to the synthesis relied on a base-catalyzed asymmetric Diels-Alder cycloaddition to establish multiple stereocenters of a highly substituted cyclohexenone unit and a facile intramolecular aza-Michael addition to form the desired bicyclic system.
中文翻译:

(+)-sarain A的功能化氮杂双环[3.3.1]壬烷部分的不对称合成方法
本文介绍了我们对不对称合成sarain A的努力,该努力导致有效制备功能化的氮杂双环[3.3.1]壬烷部分。合成的关键依赖于碱催化的不对称Diels-Alder环加成反应,以建立高度取代的环己烯酮单元和便捷的分子内氮杂-Michael加成反应的多个立体中心,从而形成所需的双环系统。


























 京公网安备 11010802027423号
京公网安备 11010802027423号