Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Set of Fe(II)-3-Hydroxyflavonolate Enzyme–Substrate Model Complexes of Atypically Coordinated Mononuclear Non-Heme Fe(II)-Dependent Quercetin 2,4-Dioxygenase
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acsomega.7b00927 Ying-Ji Sun 1 , Qian-Qian Huang 1 , Jian-Jun Zhang 1
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-18 00:00:00 , DOI: 10.1021/acsomega.7b00927 Ying-Ji Sun 1 , Qian-Qian Huang 1 , Jian-Jun Zhang 1
Affiliation
|
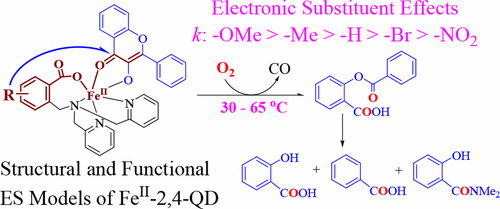
With the aim of revealing the catalytic role of atypically coordinated (3His-1Glu) active site mononuclear non-heme Fe(II)-dependent quercetin 2,4-dioxygenase (Fe-2,4-QD) and the electronic effects of the model ligands on the reactivity toward dioxygen, a set of p/m-R-substituted carboxylate-containing ligand-supported Fe(II)-3-hydroxyflavonolate complexes, [FeIILR(fla)] (LRH: 2-{[bis(pyridin-2-ylmethyl)amino]methyl}-p/m-R-benzoic acid; R: p-OMe (1), p-Me (2), m-Br (4), and m-NO2 (5); fla: 3-hydroxyflavonolate), were synthesized and characterized as structural and functional models for the ES (enzyme–substrate) complexes of Fe-2,4-QD. [FeIILR(fla)] show relatively high enzyme-type reactivity (dioxygenative ring opening of the coordinated substrate fla, single-turnover reaction) at low temperatures (30–65 °C). The reaction shows a linear Hammett plot (ρ = −1.21), and electron donating groups enhance the reaction rates. The notable difference on the reactivity can be rationalized from the electronic nature of the substituent in the ligands, which could tune the reactivity via tuning Lewis acidity of the Fe(II) ion, electron density, and the redox potential of fla. The properties and the reactivity show approximately linear correlations between λmax or E1/2 of fla and the reaction rate constant k. This work sheds light not only on understanding of electronic effects of the ligands and the property–reactivity relationship but also on the role of the catalytic reaction by Fe-2,4-QD.
中文翻译:

非协调配位单核非血红素依赖Fe(II)的槲皮素2,4-双加氧酶的Fe(II)-3-羟基黄酮酸酯酶-底物模型复合物的集合
为了揭示非典型配位的(3His-1Glu)活性位点单核非血红素依赖Fe(II)的槲皮素2,4-双加氧酶(Fe-2,4-QD)的催化作用和模型的电子效应配体对二氧的反应性,一组含p / mR取代的含羧酸盐的配体负载的Fe(II)-3-羟基黄酮酸酯配合物,[Fe II L R(fla)](L R H:2-{[bis (吡啶-2-基甲基)氨基]甲基} -p / m -R-苯甲酸; R:p- OMe(1),p -Me(2),m -Br(4)和m -NO 2(5); fla:3-羟基黄酮酸酯),已合成并表征为Fe-2,4-QD的ES(酶-底物)复合物的结构和功能模型。[Fe II L R(fla)]在低温(30–65°C)下显示出较高的酶反应性(配位基片fla的双加氧开环,单周转反应)。该反应显示线性哈米特图(ρ= -1.21),并且给电子基团提高了反应速率。可以从配体中取代基的电子性质合理化反应性上的显着差异,这可以通过调节Fe(II)离子的路易斯酸度,电子密度和fla的氧化还原电位来调节反应性。λ之间的性质和反应性显示约为线性关系最大fla的E 1/2或反应速率常数k。这项工作不仅为理解配体的电子效应和性质-反应性关系,而且为Fe-2,4-QD催化反应的作用提供了启示。
更新日期:2017-09-18
中文翻译:

非协调配位单核非血红素依赖Fe(II)的槲皮素2,4-双加氧酶的Fe(II)-3-羟基黄酮酸酯酶-底物模型复合物的集合
为了揭示非典型配位的(3His-1Glu)活性位点单核非血红素依赖Fe(II)的槲皮素2,4-双加氧酶(Fe-2,4-QD)的催化作用和模型的电子效应配体对二氧的反应性,一组含p / mR取代的含羧酸盐的配体负载的Fe(II)-3-羟基黄酮酸酯配合物,[Fe II L R(fla)](L R H:2-{[bis (吡啶-2-基甲基)氨基]甲基} -p / m -R-苯甲酸; R:p- OMe(1),p -Me(2),m -Br(4)和m -NO 2(5); fla:3-羟基黄酮酸酯),已合成并表征为Fe-2,4-QD的ES(酶-底物)复合物的结构和功能模型。[Fe II L R(fla)]在低温(30–65°C)下显示出较高的酶反应性(配位基片fla的双加氧开环,单周转反应)。该反应显示线性哈米特图(ρ= -1.21),并且给电子基团提高了反应速率。可以从配体中取代基的电子性质合理化反应性上的显着差异,这可以通过调节Fe(II)离子的路易斯酸度,电子密度和fla的氧化还原电位来调节反应性。λ之间的性质和反应性显示约为线性关系最大fla的E 1/2或反应速率常数k。这项工作不仅为理解配体的电子效应和性质-反应性关系,而且为Fe-2,4-QD催化反应的作用提供了启示。



























 京公网安备 11010802027423号
京公网安备 11010802027423号