当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Flexibility in metal–organic frameworks derived from positional and electronic effects of functional groups
CrystEngComm ( IF 3.1 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7ce00971b Hyeonbin Ha 1, 2, 3, 4 , Hyungwoo Hahm 1, 2, 3, 4 , Dong Gyun Jwa 1, 2, 3, 4 , Kwangho Yoo 1, 2, 3, 4 , Myung Hwan Park 2, 3, 4, 5 , Minyoung Yoon 4, 6, 7, 8, 9 , Youngjo Kim 1, 2, 3, 4 , Min Kim 1, 2, 3, 4
CrystEngComm ( IF 3.1 ) Pub Date : 2017-08-11 00:00:00 , DOI: 10.1039/c7ce00971b Hyeonbin Ha 1, 2, 3, 4 , Hyungwoo Hahm 1, 2, 3, 4 , Dong Gyun Jwa 1, 2, 3, 4 , Kwangho Yoo 1, 2, 3, 4 , Myung Hwan Park 2, 3, 4, 5 , Minyoung Yoon 4, 6, 7, 8, 9 , Youngjo Kim 1, 2, 3, 4 , Min Kim 1, 2, 3, 4
Affiliation
|
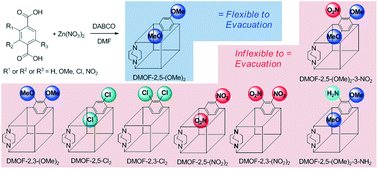
The position of identical functional groups and the subsequent electron density of structural benzene rings in a zinc-based metal–organic framework (MOF) have been controlled to reveal flexibility (or breathing behavior) differences. Both ortho- and para-positioned bi-functional benzene-1,4-dicarboxylic acid (BDC) ligands were synthesized with amino-, chloro-, methoxy-, and nitro groups. Additionally, two tri-functionalized, dimethoxy-amino and dimethoxy-nitro BDCs were prepared. All bi- and tri-functionalized BDCs were successfully incorporated into DABCO MOFs (DMOFs), except two diamino BDCs which were insoluble and thermally unstable. Among the eight bi-/tri-functionalized DMOFs, only para-dimethoxy exhibited flexibility in its framework after evacuation in preparation for N2 isotherm measurement. Since the dimethoxy combination has the most electron-rich environment in the benzene ring of the BDC in this series, this indicates that electron density plays a role in the flexibility changes of identically bi-functionalized DMOFs. However, the electron density alone could not fully explain the flexibility changes suggesting that the position of the functional groups is also important. These findings have been corroborated through the synthesis of two tri-functionalized DMOFs with identical functional group locations but opposite electronic environments.
中文翻译:

金属-有机骨架的灵活性源自官能团的位置和电子效应
在锌基金属-有机骨架(MOF)中,相同官能团的位置以及随后结构苯环的电子密度已得到控制,以显示出柔韧性(或呼吸行为)差异。两个邻位-和对位-positioned双官能苯-1,4-二羧酸(BDC)的配体用氨基- ,氯- ,甲氧基-合成,和硝基。另外,制备了两个三官能化的二甲氧基-氨基和二甲氧基-硝基BDC。除两个不溶且热不稳定的二氨基BDC外,所有双功能和三功能BDC已成功并入DABCO MOF(DMOF)。在这八个双/三官能DMOFs,只对抽空后准备制备N 2等温线时,-二甲氧基在其骨架中表现出柔性。由于该系列中BDC的苯环中的二甲氧基组合具有最富电子的环境,因此表明电子密度在相同双官能化DMOF的柔韧性变化中起作用。然而,仅电子密度不能完全解释挠性变化,表明官能团的位置也很重要。通过合成具有相同官能团位置但电子环境相反的两个三官能化DMOF,证实了这些发现。
更新日期:2017-09-18
中文翻译:

金属-有机骨架的灵活性源自官能团的位置和电子效应
在锌基金属-有机骨架(MOF)中,相同官能团的位置以及随后结构苯环的电子密度已得到控制,以显示出柔韧性(或呼吸行为)差异。两个邻位-和对位-positioned双官能苯-1,4-二羧酸(BDC)的配体用氨基- ,氯- ,甲氧基-合成,和硝基。另外,制备了两个三官能化的二甲氧基-氨基和二甲氧基-硝基BDC。除两个不溶且热不稳定的二氨基BDC外,所有双功能和三功能BDC已成功并入DABCO MOF(DMOF)。在这八个双/三官能DMOFs,只对抽空后准备制备N 2等温线时,-二甲氧基在其骨架中表现出柔性。由于该系列中BDC的苯环中的二甲氧基组合具有最富电子的环境,因此表明电子密度在相同双官能化DMOF的柔韧性变化中起作用。然而,仅电子密度不能完全解释挠性变化,表明官能团的位置也很重要。通过合成具有相同官能团位置但电子环境相反的两个三官能化DMOF,证实了这些发现。

























 京公网安备 11010802027423号
京公网安备 11010802027423号