当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
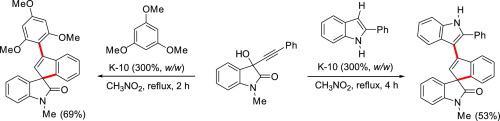
Synthesis of spiroindenyl-2-oxindoles by montmorillonite K-10-catalyzed tandem Friedel-Crafts alkenylation/hydroarylation of propargylic alcohols with sterically hindered and electron-rich arenes
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-15 , DOI: 10.1016/j.tetlet.2017.09.035 Hwa Jung Roh , Da Young Seo , Ji Yeon Ryu , Junseong Lee , Jae Nyoung Kim
中文翻译:

蒙脱土K-10催化串联Friedel-Crafts炔醇与位阻和富电子芳烃的炔丙基化/氢化芳基化反应,合成螺茚基-2-氧吲哚
更新日期:2017-09-15
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-15 , DOI: 10.1016/j.tetlet.2017.09.035 Hwa Jung Roh , Da Young Seo , Ji Yeon Ryu , Junseong Lee , Jae Nyoung Kim
|
Various spiroindenyl-2-oxindoles have been synthesized in a one-pot reaction from isatin-derived propargylic alcohols with sterically hindered and electron-rich arenes such as 2-phenylindole and 1,3,5-trimethoxybenzene. The reaction involved montmorillonite K-10-catalyzed tandem Friedel-Crafts alkenylation and a following hydroarylation of an allene intermediate.
中文翻译:

蒙脱土K-10催化串联Friedel-Crafts炔醇与位阻和富电子芳烃的炔丙基化/氢化芳基化反应,合成螺茚基-2-氧吲哚
一锅法反应中,衍生自靛红的炔丙醇与空间受阻且富电子的芳烃(例如2-苯基吲哚和1,3,5-三甲氧基苯)以一锅法合成了各种螺茚基-2-氧吲哚。该反应涉及蒙脱石K-10-催化的串联Friedel-Crafts烯基化和随后的烯丙基中间体的氢芳基化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号