Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computationally Mapping pKa Shifts Due to the Presence of a Polyelectrolyte Chain around Whey Proteins
Langmuir ( IF 3.9 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.langmuir.7b02271 Deepti Srivastava 1 , Erik Santiso 1 , Keith Gubbins 1 , Fernando Luís Barroso da Silva 1, 2
Langmuir ( IF 3.9 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.langmuir.7b02271 Deepti Srivastava 1 , Erik Santiso 1 , Keith Gubbins 1 , Fernando Luís Barroso da Silva 1, 2
Affiliation
|
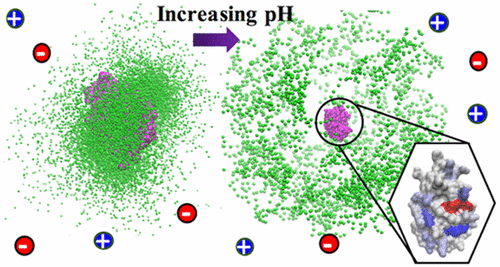
Experimental studies have shown the formation of soluble complexes in the pure repulsive Coulombic regime even when the net charges of the protein and the polyelectrolyte have the same sign (De Kruif et al. Curr. Opin. Colloid Interface Sci. 2004, 9, 340; De Vries et al. J. Chem. Phys. 2003, 118, 4649; Grymonpre et al. Biomacromolecules 2001, 2, 422; Hattori et al. Langmuir 2000, 16, 9738). This attractive phenomenon has often been described as “complexation on the wrong side of pI”. While one theory assumes the existence of “charged patches” on the protein surface from ion–dipole interactions, thus allowing a polyelectrolyte to bind to an oppositely heterogeneous charged protein region, another theoretical view considers the induced-charge interactions to be the dominant factor in these complexations. This charge regulation mechanism can be described by proton fluctuations resulting from mutual rearrangements of the distributions of the charged groups, due to perturbations of the acid–base equilibrium. Using constant-pH Monte Carlo simulations and several quantitative and visual analysis tools, we investigate the significance of each of these interactions for two whey proteins, α-lactalbumin (α-LA) and lysozyme (LYZ). Through physical chemistry parameters, free energies of interactions, and the mapping of amino acid pKa shifts and polyelectrolyte trajectories, we show the charge regulation mechanism to be the most important contributor in protein-polyelectrolyte complexation regardless of pH, dipole moment, and protein capacitance in a low salt regime.
中文翻译:

由于乳清蛋白周围存在聚电解质链,计算计算p K a位移
实验研究表明,即使蛋白质和聚电解质的净电荷具有相同的符号,在纯排斥性库仑溶液中也会形成可溶性复合物(De Kruif等人, Curr.Opin.Colloid Interface Sci。 2004年, 9, 340; De Vries等。 J.化学。物理 2003年, 118, 4649; Grymonpre等。 生物大分子 2001, 2个, 422; Hattori等。 朗缪尔 2000, 16, 9738)。这种吸引人的现象通常被描述为“ pI错误一侧的复杂化”。虽然一种理论认为离子-偶极相互作用在蛋白质表面存在“带电斑块”,从而使聚电解质与相反的异质带电蛋白质区域结合,但另一种理论观点则认为诱导电荷相互作用是蛋白质中的主要因素。这些复杂的事情。这种电荷调节机制可以通过质子波动来描述,质子波动是由于酸碱平衡的扰动而导致的,由于带电基团的分布相互重新排列而引起的。使用恒定pH值的蒙特卡洛模拟以及几种定量和视觉分析工具,我们研究了两种乳清蛋白α-乳清蛋白(α-LA)和溶菌酶(LYZ)相互作用的重要性。K a位移和聚电解质的轨迹,我们表明电荷调节机制是蛋白质-聚电解质络合中最重要的贡献者,而与pH,偶极矩和低盐状态下的蛋白质电容无关。
更新日期:2017-09-15
中文翻译:

由于乳清蛋白周围存在聚电解质链,计算计算p K a位移
实验研究表明,即使蛋白质和聚电解质的净电荷具有相同的符号,在纯排斥性库仑溶液中也会形成可溶性复合物(De Kruif等人, Curr.Opin.Colloid Interface Sci。



























 京公网安备 11010802027423号
京公网安备 11010802027423号