当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
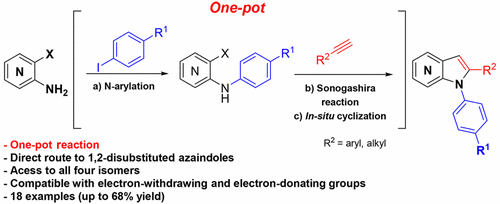
One-Pot Synthesis of 1,2-Disubstituted 4-, 5-, 6-, and 7-Azaindoles from Amino-o-halopyridines via N-Arylation/Sonogashira/Cyclization Reaction
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.orglett.7b02403 Sara I. Purificação 1 , Marina J. D. Pires 1 , Rafael Rippel 1 , A. Sofia Santos 1 , M. Manuel B. Marques 1
Organic Letters ( IF 5.2 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.orglett.7b02403 Sara I. Purificação 1 , Marina J. D. Pires 1 , Rafael Rippel 1 , A. Sofia Santos 1 , M. Manuel B. Marques 1
Affiliation
|
A direct synthesis of several 1,2-disubstituted 4-, 5-, 6-, and 7-azaindoles from available amino-o-halopyridines is described. This procedure involves a palladium-catalyzed N-arylation followed by a Sonogashira reaction and subsequent cyclization in a one-pot manner, exhibiting a wide scope and compatibility with electron-withdrawing and electron-donating groups. The strategy represents an advancement in azaindole chemistry with a straightforward approach toward 1,2-disubstituted azaindoles, while avoiding complex N-arylations of hindered 2-substituted azaindoles and difficult purification steps of intermediates.
中文翻译:

氨基邻卤代吡啶通过N-芳基化/ Sonogashira /环化反应一锅法合成1,2-二取代的4-,5-,6-和7-氮杂吲哚
若干直接合成1,2-二取代的4-,5-,6-,从可用氨基和7-氮杂吲哚ö -halopyridines进行说明。该方法涉及钯催化的N-芳基化,然后进行Sonogashira反应,随后以一锅法进行环化,表现出广泛的范围并且与吸电子和供电子基团相容。该策略代表了直接向1,2-二取代的吲哚吲哚类化合物迈进的吲哚化学的进步,同时避免了受阻的2-取代的吲哚类化合物的复杂N-芳基化和中间体的困难纯化步骤。
更新日期:2017-09-15
中文翻译:

氨基邻卤代吡啶通过N-芳基化/ Sonogashira /环化反应一锅法合成1,2-二取代的4-,5-,6-和7-氮杂吲哚
若干直接合成1,2-二取代的4-,5-,6-,从可用氨基和7-氮杂吲哚ö -halopyridines进行说明。该方法涉及钯催化的N-芳基化,然后进行Sonogashira反应,随后以一锅法进行环化,表现出广泛的范围并且与吸电子和供电子基团相容。该策略代表了直接向1,2-二取代的吲哚吲哚类化合物迈进的吲哚化学的进步,同时避免了受阻的2-取代的吲哚类化合物的复杂N-芳基化和中间体的困难纯化步骤。


























 京公网安备 11010802027423号
京公网安备 11010802027423号