PLOS Neglected Tropical Diseases ( IF 3.8 ) Pub Date : 2017-09-15 , DOI: 10.1371/journal.pntd.0005932 Joar Pinto , Steven Odongo , Felicity Lee , Vaiva Gaspariunaite , Serge Muyldermans , Stefan Magez , Yann G.-J. Sterckx
|
Background
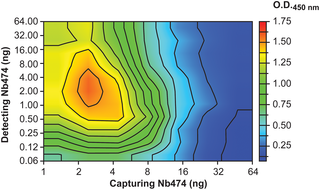
Animal African trypanosomosis (AAT) is a neglected tropical disease which imposes a heavy burden on the livestock industry in Sub-Saharan Africa. Its causative agents are Trypanosoma parasites, with T. congolense and T. vivax being responsible for the majority of the cases. Recently, we identified a Nanobody (Nb474) that was employed to develop a homologous sandwich ELISA targeting T. congolense fructose-1,6-bisphosphate aldolase (TcoALD). Despite the high sequence identity between trypanosomatid aldolases, the Nb474-based immunoassay is highly specific for T. congolense detection. The results presented in this paper yield insights into the molecular principles underlying the assay’s high specificity.
Methodology/Principal findings
The structure of the Nb474-TcoALD complex was determined via X-ray crystallography. Together with analytical gel filtration, the structure reveals that a single TcoALD tetramer contains four binding sites for Nb474. Through a comparison with the crystal structures of two other trypanosomatid aldolases, TcoALD residues Ala77 and Leu106 were identified as hot spots for specificity. Via ELISA and surface plasmon resonance (SPR), we demonstrate that mutation of these residues does not abolish TcoALD recognition by Nb474, but does lead to a lack of detection in the Nb474-based homologous sandwich immunoassay.
Conclusions/Significance
The results show that the high specificity of the Nb474-based immunoassay is not determined by the initial recognition event between Nb474 and TcoALD, but rather by its homologous sandwich design. This (i) provides insights into the optimal set-up of the assay, (ii) may be of great significance for field applications as it could explain the potential detection escape of certain T. congolense strains, and (iii) may be of general interest to those developing similar assays.
中文翻译:

针对糖体醛缩酶的锥虫锥虫免疫测定的高特异性的结构基础
背景
非洲动物锥虫病(AAT)是一种被忽视的热带病,对撒哈拉以南非洲的畜牧业构成沉重负担。其病原体是锥虫锥虫,T。congolense和Ť。大部分案件由vivax负责。最近,我们鉴定了一种纳米抗体(Nb474),该抗体被用于开发针对T的同源夹心ELISA 。congolense -1,6-双磷酸果糖醛缩酶(Tco ALD)。尽管锥虫醛糖醛缩醛缩醛酶之间具有很高的序列同一性,但基于Nb474的免疫测定法对T具有高度特异性。共犯检测。本文介绍的结果可深入了解测定法高特异性的分子原理。
方法/主要发现
Nb474- Tco ALD复合物的结构是通过X射线晶体学确定的。连同分析性凝胶过滤一起,该结构揭示单个Tco ALD四聚体包含Nb474的四个结合位点。通过与其他两种锥虫性醛缩酶的晶体结构比较,Tco ALD残基Ala77和Leu106被鉴定为特异性热点。通过ELISA和表面等离振子共振(SPR),我们证明了这些残基的突变不会废除Nb474对Tco ALD的识别,但会导致基于Nb474的同源夹心免疫测定法中检测不到。
结论/意义
结果表明,基于Nb474的免疫测定的高特异性不是由Nb474和Tco ALD之间的初始识别事件决定的,而是由其同源夹心设计决定的。这(i)提供了对最佳检测方法的见解,(ii)对于现场应用可能具有重要意义,因为它可以解释某些T的潜在检测逃逸。congolense菌株,和(iii)可以是一般的感兴趣的那些开发类似的测定法。

























 京公网安备 11010802027423号
京公网安备 11010802027423号