当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Charge-transfer-energy-dependent oxygen evolution reaction mechanisms for perovskite oxides
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7ee02052j Wesley T. Hong 1, 2, 3, 4 , Kelsey A. Stoerzinger 1, 2, 3, 4 , Yueh-Lin Lee 2, 3, 4, 5 , Livia Giordano 2, 3, 4, 5, 6 , Alexis Grimaud 2, 3, 4, 5 , Alyssa M. Johnson 4, 7, 8, 9 , Jonathan Hwang 1, 2, 3, 4 , Ethan J. Crumlin 4, 10, 11, 12 , Wanli Yang 4, 10, 11, 12 , Yang Shao-Horn 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2017-09-04 00:00:00 , DOI: 10.1039/c7ee02052j Wesley T. Hong 1, 2, 3, 4 , Kelsey A. Stoerzinger 1, 2, 3, 4 , Yueh-Lin Lee 2, 3, 4, 5 , Livia Giordano 2, 3, 4, 5, 6 , Alexis Grimaud 2, 3, 4, 5 , Alyssa M. Johnson 4, 7, 8, 9 , Jonathan Hwang 1, 2, 3, 4 , Ethan J. Crumlin 4, 10, 11, 12 , Wanli Yang 4, 10, 11, 12 , Yang Shao-Horn 1, 2, 3, 4, 5
Affiliation
|
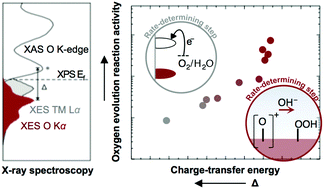
Numerous studies have reported electronic activity descriptors of oxygen evolution reaction (OER) for oxide catalysts under a single reaction mechanism. However, recent works have revealed that a single mechanism is not at play across oxide chemistries. These works underscore a need to deeply investigate the electronic structure details of active oxide catalysts and how they align with the OER potential, which is critical to understanding the interfacial charge-transfer kinetics that dictate catalytic mechanisms. In this work, we use soft X-ray emission and absorption spectroscopy of perovskites to analyze the partial density of states on an absolute energy scale, from which energetic barriers for electron transfer and surface deprotonation were estimated and correlated with OER activity. Through this lens, we identify that decreasing the solid-state charge-transfer energy of perovskites can change the mechanisms of the OER from electron-transfer-limited to proton–electron-coupled, to proton-transfer-limited reactions. This concept is supported by the analysis of potential energy surfaces for sequential and concerted proton–electron transfer pathways using a Marcus model. Our work highlights the importance of understanding the physical origin of experimental OER activity trends with electronic descriptors and the need to promote surface deprotonation from oxides to discover new catalysts with enhanced activity.
中文翻译:

钙钛矿氧化物的电荷转移能量依赖性氧释放反应机理
大量研究报告了在单一反应机理下氧化物催化剂的氧释放反应(OER)的电子活性描述符。但是,最近的研究表明,跨氧化物化学没有单一机制起作用。这些工作强调需要深入研究活性氧化物催化剂的电子结构细节以及它们如何与OER势能对齐,这对于理解决定催化机理的界面电荷转移动力学至关重要。在这项工作中,我们使用钙钛矿的软X射线发射和吸收光谱在绝对能级上分析状态的部分密度,由此估算出电子转移和表面去质子化的能垒,并与OER活性相关。通过这个镜头,我们发现,降低钙钛矿的固态电荷转移能可以改变OER的机理,从电子转移受限的反应到质子-电子耦合,再到质子转移受限的反应。使用Marcus模型对连续和协调的质子-电子转移路径的势能面的分析支持了这一概念。我们的工作突显了用电子描述符理解实验OER活性趋势的物理成因的重要性,以及促进氧化物表面去质子化以发现具有增强活性的新型催化剂的需要。使用Marcus模型对连续和协调的质子-电子转移路径的势能面的分析支持了这一概念。我们的工作突显了用电子描述符理解实验OER活性趋势的物理成因的重要性,以及促进氧化物表面去质子化以发现具有增强活性的新型催化剂的需要。使用Marcus模型对连续和协调的质子-电子转移路径的势能面的分析支持了这一概念。我们的工作突显了用电子描述符理解实验OER活性趋势的物理成因的重要性,以及促进氧化物表面去质子化以发现具有增强活性的新型催化剂的需要。
更新日期:2017-09-15
中文翻译:

钙钛矿氧化物的电荷转移能量依赖性氧释放反应机理
大量研究报告了在单一反应机理下氧化物催化剂的氧释放反应(OER)的电子活性描述符。但是,最近的研究表明,跨氧化物化学没有单一机制起作用。这些工作强调需要深入研究活性氧化物催化剂的电子结构细节以及它们如何与OER势能对齐,这对于理解决定催化机理的界面电荷转移动力学至关重要。在这项工作中,我们使用钙钛矿的软X射线发射和吸收光谱在绝对能级上分析状态的部分密度,由此估算出电子转移和表面去质子化的能垒,并与OER活性相关。通过这个镜头,我们发现,降低钙钛矿的固态电荷转移能可以改变OER的机理,从电子转移受限的反应到质子-电子耦合,再到质子转移受限的反应。使用Marcus模型对连续和协调的质子-电子转移路径的势能面的分析支持了这一概念。我们的工作突显了用电子描述符理解实验OER活性趋势的物理成因的重要性,以及促进氧化物表面去质子化以发现具有增强活性的新型催化剂的需要。使用Marcus模型对连续和协调的质子-电子转移路径的势能面的分析支持了这一概念。我们的工作突显了用电子描述符理解实验OER活性趋势的物理成因的重要性,以及促进氧化物表面去质子化以发现具有增强活性的新型催化剂的需要。使用Marcus模型对连续和协调的质子-电子转移路径的势能面的分析支持了这一概念。我们的工作突显了用电子描述符理解实验OER活性趋势的物理成因的重要性,以及促进氧化物表面去质子化以发现具有增强活性的新型催化剂的需要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号