当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper‐Catalyzed Regioselective Formal Allylboration of Aryl‐Substituted Terminal Alkynes with Bis(pinacolato)diboron and Allyl Halides/Sulfonates
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-06-29 , DOI: 10.1002/ajoc.201700263 Haitong Jing 1 , Xiaorui Feng 1 , Minjie Guo 2 , Sen Zhou 1 , Yanlin Li 1 , Jiacheng Zhang 3 , Wentao Zhao 1 , Xiangyang Tang 1 , Guangwei Wang 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-06-29 , DOI: 10.1002/ajoc.201700263 Haitong Jing 1 , Xiaorui Feng 1 , Minjie Guo 2 , Sen Zhou 1 , Yanlin Li 1 , Jiacheng Zhang 3 , Wentao Zhao 1 , Xiangyang Tang 1 , Guangwei Wang 1
Affiliation
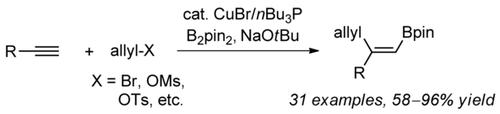
|
A copper‐catalyzed formal allylboration of terminal alkynes using allyl bromides or sulfonates as electrophiles has been achieved. This method exhibits good regio‐/stereoselectivity and good functional‐group tolerance for various substituents on the aromatic rings of arylethynes. Through this strategy, various trisubstituted vinyl pinacol boronic esters with a 1,4‐diene skeleton have been constructed. In order to demonstrate the utility of this method, the resulting 1,4‐dienes with boron substitution were further used in transformations such as Suzuki coupling, oxidation, halogenation, and so on, leading to various important compounds.
中文翻译:

铜催化的芳基取代的末端炔烃与双(频哪醇)二硼和烯丙基卤化物/磺酸盐的区域选择性形式烯丙基硼化
使用烯丙基溴或磺酸盐作为亲电子试剂,实现了末端炔烃的铜催化形式的烯丙基硼化。该方法对芳乙炔芳环上的各种取代基具有良好的区域选择性/立体选择性和对官能团的耐受性。通过这种策略,已经构造了各种具有1,4-二烯骨架的三取代乙烯基频哪醇硼酸酯。为了证明该方法的实用性,将所得的具有硼取代基的1,4-二烯进一步用于诸如Suzuki偶联,氧化,卤化等转化中,从而生成各种重要的化合物。
更新日期:2017-06-29
中文翻译:

铜催化的芳基取代的末端炔烃与双(频哪醇)二硼和烯丙基卤化物/磺酸盐的区域选择性形式烯丙基硼化
使用烯丙基溴或磺酸盐作为亲电子试剂,实现了末端炔烃的铜催化形式的烯丙基硼化。该方法对芳乙炔芳环上的各种取代基具有良好的区域选择性/立体选择性和对官能团的耐受性。通过这种策略,已经构造了各种具有1,4-二烯骨架的三取代乙烯基频哪醇硼酸酯。为了证明该方法的实用性,将所得的具有硼取代基的1,4-二烯进一步用于诸如Suzuki偶联,氧化,卤化等转化中,从而生成各种重要的化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号