当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper/Bisphosphine Catalysts in the Internally Borylative Aminoboration of Unactivated Terminal Alkenes with Bis(pinacolato)diboron
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.joc.7b01874 Kodai Kato 1 , Koji Hirano 1 , Masahiro Miura 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-15 00:00:00 , DOI: 10.1021/acs.joc.7b01874 Kodai Kato 1 , Koji Hirano 1 , Masahiro Miura 1
Affiliation
|
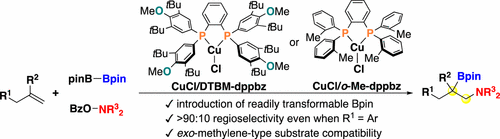
Cu(I)/modified dppbz catalyst systems for the regioselective aminoboration of unactivated terminal alkenes have been developed. The bisphosphine-based Cu catalysis enables the introduction of the readily transformable Bpin group at the more congested internal position and shows better regioselectivity for broader terminal alkenes involving sterically demanding allylbenzenes, which are relatively challenging substrates in the previous IPrCuBr catalysis. Additionally, the second-generation catalyst systems accommodate the exo-methylene-type disubstituted alkenes to deliver the corresponding aminoborated products in good yields with a high regioselectivity.
中文翻译:

铜/双膦催化剂在未活化的末端烯烃与双(频哪醇)双硼的内部硼酸化氨基硼化中的催化作用
已开发出用于未活化末端烯烃的区域选择性氨基硼化的Cu(I)/改性dppbz催化剂体系。基于双膦的Cu催化能够在内部更拥挤的位置引入易于转化的Bpin基团,并且对涉及空间需求的烯丙基苯的较宽的末端烯烃表现出更好的区域选择性,而烯丙基苯是先前IPrCuBr催化中相对具有挑战性的底物。另外,第二代催化剂体系容纳外-亚甲基型双取代烯烃,以高收率和高区域选择性递送相应的氨基硼代产物。
更新日期:2017-09-15
中文翻译:

铜/双膦催化剂在未活化的末端烯烃与双(频哪醇)双硼的内部硼酸化氨基硼化中的催化作用
已开发出用于未活化末端烯烃的区域选择性氨基硼化的Cu(I)/改性dppbz催化剂体系。基于双膦的Cu催化能够在内部更拥挤的位置引入易于转化的Bpin基团,并且对涉及空间需求的烯丙基苯的较宽的末端烯烃表现出更好的区域选择性,而烯丙基苯是先前IPrCuBr催化中相对具有挑战性的底物。另外,第二代催化剂体系容纳外-亚甲基型双取代烯烃,以高收率和高区域选择性递送相应的氨基硼代产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号