PLoS Pathogens ( IF 6.7 ) Pub Date : 2017-09-14 , DOI: 10.1371/journal.ppat.1006569 Derek B. Danahy , Scott M. Anthony , Isaac J. Jensen , Stacey M. Hartwig , Qiang Shan , Hai-Hui Xue , John T. Harty , Thomas S. Griffith , Vladimir P. Badovinac
|
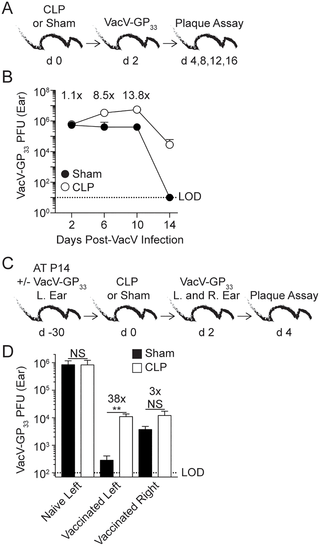
Sepsis is a systemic infection that enhances host vulnerability to secondary infections normally controlled by T cells. Using CLP sepsis model, we observed that sepsis induces apoptosis of circulating memory CD8 T-cells (TCIRCM) and diminishes their effector functions, leading to impaired CD8 T-cell mediated protection to systemic pathogen re-infection. In the context of localized re-infections, tissue resident memory CD8 T-cells (TRM) provide robust protection in a variety of infectious models. TRM rapidly ‘sense’ infection in non-lymphoid tissues and ‘alarm’ the host by enhancing immune cell recruitment to the site of the infection to accelerate pathogen clearance. Here, we show that compared to pathogen-specific TCIRCM, sepsis does not invoke significant numerical decline of Vaccinia virus induced skin-TRM keeping their effector functions (e.g., Ag-dependent IFN-γ production) intact. IFN-γ-mediated recruitment of immune cells to the site of localized infection was, however, reduced in CLP hosts despite TRM maintaining their ‘sensing and alarming’ functions. The capacity of memory CD8 T-cells in the septic environment to respond to inflammatory cues and arrive to the site of secondary infection/antigen exposure remained normal suggesting T-cell-extrinsic factors contributed to the observed lesion. Mechanistically, we showed that IFN-γ produced rapidly during sepsis-induced cytokine storm leads to reduced IFN-γR1 expression on vascular endothelium. As a consequence, decreased expression of adhesion molecules and/or chemokines (VCAM1 and CXCL9) on skin endothelial cells in response to TRM-derived IFN-γ was observed, leading to sub-optimal bystander-recruitment of effector cells and increased susceptibility to pathogen re-encounter. Importantly, as visualized by intravital 2-photon microscopy, exogenous administration of CXCL9/10 was sufficient to correct sepsis-induced impairments in recruitment of effector cells at the localized site of TRM antigen recognition. Thus, sepsis has the capacity to alter skin TRM anamnestic responses without directly impacting TRM number and/or function, an observation that helps to further define the immunoparalysis phase in sepsis survivors.
中文翻译:

尽管皮肤常驻记忆CD8 T细胞具有最佳的感知和报警功能,但细菌性败血症仍可削弱旁观者募集效应细胞至感染的皮肤
脓毒症是一种全身感染,可增强宿主对通常由T细胞控制的继发感染的脆弱性。使用CLP脓毒症模型,我们观察到脓毒症可诱导循环记忆CD8 T细胞(T CIRCM)凋亡并减弱其效应功能,从而导致CD8 T细胞介导的对系统性病原体再感染的保护受损。在局部再感染的情况下,组织常驻记忆CD8 T细胞(T RM)在多种感染模型中提供强大的保护。T RM通过增强免疫细胞募集到感染部位来加速病原体清除,从而在非淋巴组织中迅速“感知”感染,并“警告”宿主。在这里,我们证明了与病原体特异性T CIRCM相比,败血症没有引起痘苗病毒诱导的皮肤-T RM的显着数值下降,从而保持其效应子功能(例如,Ag依赖性IFN-γ产生)保持完整。尽管有T RM,但在CLP宿主中,IFN-γ介导的免疫细胞募集到局部感染的部位却减少了保持其“感应和报警”功能。在脓毒症环境中,记忆CD8 T细胞对炎症信号作出反应并到达继发感染/抗原暴露部位的能力仍然正常,这表明T细胞外源性因素促成了观察到的病变。从机制上讲,我们显示在脓毒症诱导的细胞因子风暴期间迅速产生的IFN-γ导致血管内皮上IFN-γR1的表达降低。因此,响应T RM,皮肤内皮细胞上粘附分子和/或趋化因子(VCAM1和CXCL9)的表达降低。观察到源自IFN-γ的细胞,导致效应细胞的次优招募,并增加了对病原体再遇到的敏感性。重要的是,通过活体2光子显微镜观察,CXCL9 / 10的外源给药足以纠正败血症诱导的TRM抗原识别定位位点效应细胞募集的损伤。因此,脓毒症具有改变皮肤T RM记忆消除反应的能力,而没有直接影响T RM数量和/或功能,这一观察有助于进一步确定脓毒症幸存者的免疫麻痹阶段。



























 京公网安备 11010802027423号
京公网安备 11010802027423号