European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.ejmech.2017.09.015 Guoshun Luo , Xinyu Li , Guoqing Zhang , Chengzhe Wu , Zhengpu Tang , Linyi Liu , Qidong You , Hua Xiang
|
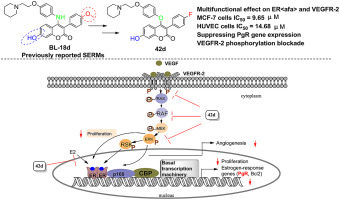
There is considerable interest in developing new SERMs as multifunctional agents in women's health. Development of dual selective estrogen receptor modulators/VEGFR-2 inhibitors (SERMs/V-2I) has been an attractive strategy for the discovery of new breast cancer therapeutic agents. Our previous efforts led to the preparation of a series of 3-aryl-4-anilino-2H-chromen-2-ones endowed with potent estrogen receptor binding affinity and anti-proliferative efficacy. In this study, various structurally related 3-aryl-4-anilino/aryloxy-2H-chromen-2-one analogues were rationally designed, synthesized and evaluated as a new chemo-type of dual ERα and VEGFR-2 inhibitors. Most of the derivatives exhibited potent activities in both enzymatic and cellular assays. SAR investigation revealed that introducing of bioisosteric O atom at the C-4 position of coumarin scaffold is beneficial to improve the inhibitory potency, especially in ERα binding affinity assay. Furthermore, most of the piperidyl substituted compounds showed better inhibitory activity against MCF-7 and Ishikawa cells than lead compounds BL-18d, tamoxifen and Vandetanib. Optimization of the hit compound, identified in an ERα binding affinity assay, led to compound 42d, exhibiting an IC50 for ERα binding affinity of 2.19 μM while retaining an excellent inhibition on VGFR-2 as well as a potent suppression on the growth of angiogenesis-related cells. In RT-PCR assay, 42d exerted significantly antiestrogenic property via suppressing the expression of progesterone receptor (PgR) mRNA in MCF-7 cells, which was consistent with the ERα antagonistic property of a selective estrogen receptor modulator. Further mechanism investigation demonstrated that compound 42d could inhibit the activation of VEGFR-2 and subsequent signaling transduction of Raf-1/MAPK/ERK pathway in MCF-7 cells. All these results together with molecular modeling studies open a new avenue for the development of multifunctional agents targeting ERα and VEGFR-2 in the therapy of some breast cancers.
中文翻译:

基于3-芳基-4-芳氧基-2H-铬-2-基骨架的新型SERM-双重ERα/ VEGFR-2配体治疗乳腺癌的可能方法
开发新的SERM作为妇女健康的多功能制剂引起了人们的极大兴趣。双重选择性雌激素受体调节剂/ VEGFR-2抑制剂(SERMs / V-2I)的开发一直是发现新型乳腺癌治疗剂的有吸引力的策略。我们先前的努力导致了一系列具有强大的雌激素受体结合亲和力和抗增殖功效的3-芳基-4-苯胺基-2H-铬2-2-酮的制备。在这项研究中,各种结构上相关的3-芳基-4-苯胺基/芳氧基-2H-铬-2-基类似物被合理设计,合成和评估为新型化学型的双重ERα和VEGFR-2抑制剂。大多数衍生物在酶和细胞分析中均显示出有效的活性。SAR调查显示,在香豆素支架的C-4位引入生物等位O原子有利于提高抑制能力,特别是在ERα结合亲和力测定中。此外,大多数哌啶基取代的化合物对MCF-7和Ishikawa细胞的抑制活性均优于先导化合物BL-18d,他莫昔芬和Vandetanib。在ERα结合亲和力测定中确定的最佳命中化合物导致化合物42d表现出2.19μM的ERα结合亲和力IC 50,同时保留了对VGFR-2的优异抑制作用以及对血管生成生长的有效抑制作用相关细胞。在RT-PCR分析中,42d通过抑制MCF-7细胞中的孕激素受体(PgR)mRNA的表达发挥了显着的抗雌激素作用,这与选择性雌激素受体调节剂的ERα拮抗作用相一致。进一步的机理研究表明,化合物42d可以抑制MCF-7细胞中VEGFR-2的激活和Raf-1 / MAPK / ERK通路的信号转导。所有这些结果以及分子建模研究为在某些乳腺癌的治疗中开发针对ERα和VEGFR-2的多功能药物开辟了新途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号