当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of potent antiviral (HSV-1) quinazolinones and initial structure-activity relationship studies
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.bmcl.2017.09.026 Carla E. Brown , Tiffany Kong , James McNulty , Leonardo D'Aiuto , Kelly Williamson , Lora McClain , Paolo Piazza , Vishwajit L. Nimgaonkar
中文翻译:

高效抗病毒(HSV-1)喹唑啉酮的发现和初步构效关系研究
更新日期:2017-09-14
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2017-09-14 , DOI: 10.1016/j.bmcl.2017.09.026 Carla E. Brown , Tiffany Kong , James McNulty , Leonardo D'Aiuto , Kelly Williamson , Lora McClain , Paolo Piazza , Vishwajit L. Nimgaonkar
|
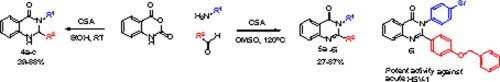
The discovery of antiviral activity of 2,3-disubstituted quinazolinones, prepared by a one-pot, three-component condensation of isatoic anhydride with amines and aldehydes, against Herpes Simplex Virus (HSV)-1 is reported. Sequential iterative synthesis/antiviral assessment allowed structure-activity relationship (SAR) generation revealing synergistic structural features required for potent anti-HSV-1 activity. The most potent derivatives show greater efficacy than acyclovir against acute HSV-1 infections in neurons and minimal toxicity to the host.
中文翻译:

高效抗病毒(HSV-1)喹唑啉酮的发现和初步构效关系研究
据报道,发现由2,3-二取代的喹唑啉酮类化合物通过一锅三组分的等位酸酐与胺和醛的缩合制备而成的抗单纯疱疹病毒(HSV)-1的抗病毒活性。顺序的迭代合成/抗病毒评估允许结构-活性关系(SAR)生成,揭示有效的抗HSV-1活性所需的协同结构特征。与阿昔洛韦相比,最有效的衍生物在神经元中对急性HSV-1感染的疗效优于阿昔洛韦,对宿主的毒性也最小。



























 京公网安备 11010802027423号
京公网安备 11010802027423号