JAMA Oncology ( IF 28.4 ) Pub Date : 2017-09-01 , DOI: 10.1001/jamaoncol.2017.0041 Gaurav Goyal 1 , Mithun V Shah 1 , Timothy G Call 1 , Mark R Litzow 1 , William J Hogan 1 , Ronald S Go 1
|
Importance While cladribine is best known for the treatment of hairy cell leukemia and other lymphoid cancers, it also has activity against myeloid neoplasms, such as Erdheim-Chester disease (ECD).
Objective To assess the efficacy of cladribine (2-chloro-2′-deoxyadenosine) in the treatment of ECD.
Design, Setting, and Participants This study was a single-institution retrospective medical record review from January 1, 1998, to April 6, 2016, at a tertiary academic medical center. In all eligible cases, the diagnosis of ECD was made using clinical criteria in conjunction with histopathologic findings.
Exposure Cladribine therapy in first-line treatment or later.
Main Outcomes and Measures Two response criteria were used, clinical and radiological. For clinical response, the following criteria were used: complete response (complete resolution of symptoms attributed to ECD), partial response (partial resolution of symptoms attributed to ECD), stable disease (no change in symptoms attributed to ECD), and progressive disease (worsening of symptoms attributed to ECD). For radiological response, the following categories were used: complete response (complete resolution of proven or suspected lesion due to ECD), partial response (partial resolution of proven or suspected lesion due to ECD), stable disease (no significant change in proven or suspected lesion due to ECD for ≥3 months), and progressive disease (progression or worsening of proven or suspected lesion due to ECD).
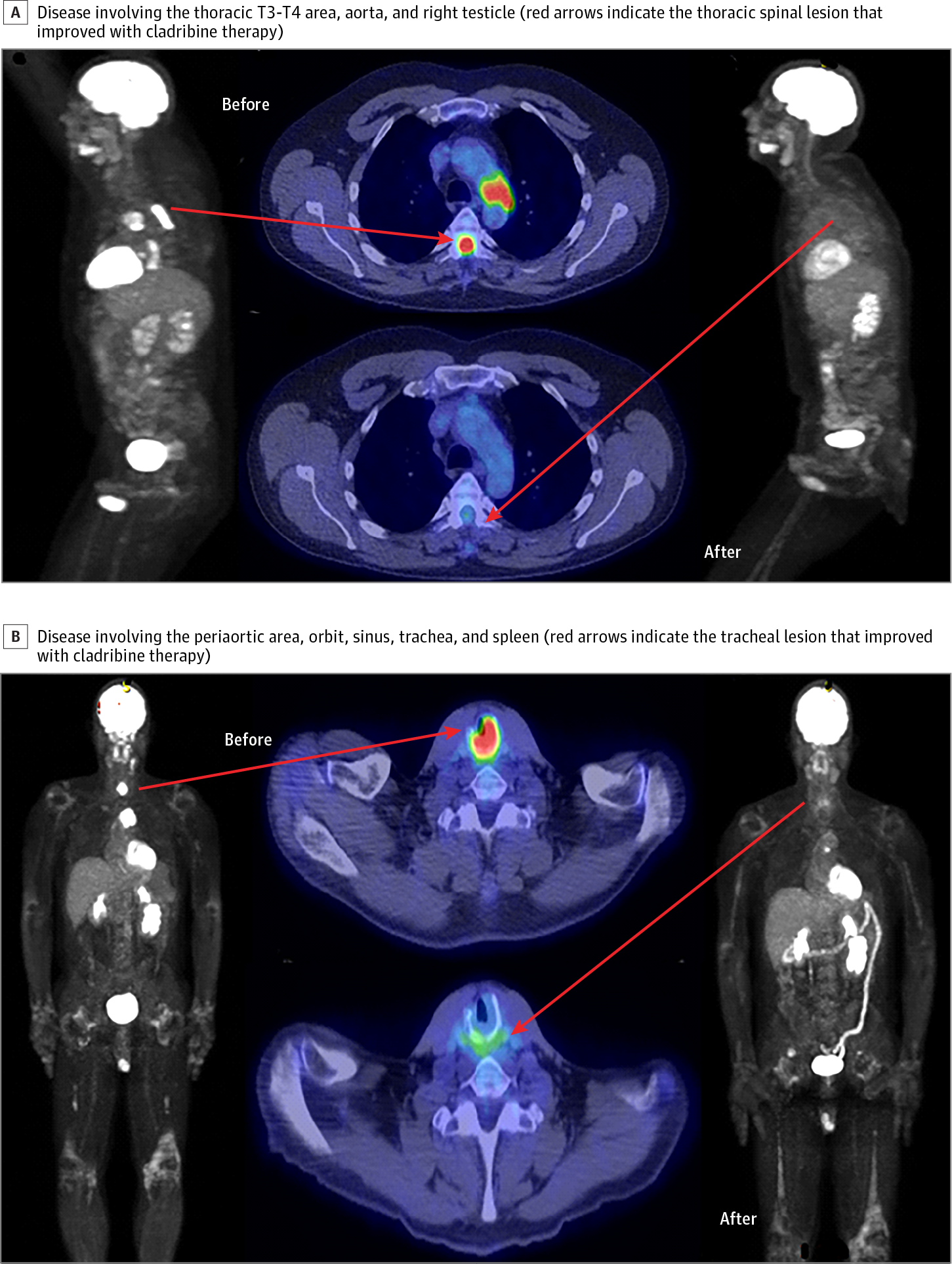
Results A total of 63 adult patients with confirmed ECD were identified. Their median age at diagnosis of ECD was 54 years (age range, 18-80 years), and 67% (42 of 63) were male. Cladribine was the most commonly used chemotherapeutic agent and was administered in 21 of 63 patients (33%). Their median age at the time of cladribine therapy was 62 years (age range, 40-78 years). Cladribine was used as the first-line treatment in 9 patients and as later-line treatment in the remaining 12 patients. The median number of cycles of cladribine administered was 2.5 (range, 1-6). The overall clinical response rate was 52% (9 of 17) (6% [1 of 17] complete response and 46% [8 of 17] partial response), with 18% (3 of 17) stable disease and 30% (5 of 17) progressive disease. Among patients who responded to cladribine therapy, the median duration of clinical response was 9 months (range, 6-129 months), with ongoing response in 2 patients. The overall radiological response rate was 54% (8 of 15) (all partial response), with 26% (4 of 15) stable disease and 20% (2 of 15) progressive disease. Treatment-related adverse effects included 2 infectious complications (pneumonia and central line infection, both requiring hospitalization) and 2 hematologic adverse effects (grade 4 neutropenia and thrombocytopenia, and grade 3 neutropenia, both requiring therapy discontinuation).
Conclusions and Relevance Cladribine has moderate clinical efficacy in the treatment of ECD and can be considered a treatment option in cases without the BRAF V600E mutation. It is generally well tolerated and may result in a durable response.
中文翻译:

克拉屈滨治疗 Erdheim-Chester 病的临床和放射学反应
重要性 虽然克拉屈滨以治疗毛细胞白血病和其他淋巴癌而闻名,但它也具有抗髓系肿瘤的活性,例如 Erdheim-Chester 病 (ECD)。
目的 评价克拉屈滨(2-氯-2'-脱氧腺苷)治疗ECD的疗效。
设计、设置和参与者 本研究是 1998 年 1 月 1 日至 2016 年 4 月 6 日在一家三级学术医疗中心进行的单机构回顾性医疗记录审查。在所有符合条件的病例中,ECD 的诊断是根据临床标准结合组织病理学结果进行的。
在一线或以后的治疗中暴露克拉屈滨治疗。
主要结果和措施 使用了两个响应标准,临床和放射学。对于临床反应,使用以下标准:完全反应(归因于 ECD 的症状完全消退)、部分反应(归因于 ECD 的症状部分消退)、疾病稳定(归因于 ECD 的症状没有变化)和疾病进展(归因于 ECD 的症状恶化)。对于放射学反应,使用以下类别:完全反应(由于 ECD 导致的已证实或疑似病变完全消退)、部分反应(由于 ECD 导致已证实或疑似病变部分消退)、疾病稳定(已证实或疑似病变没有显着变化) ECD 引起的病变持续 ≥3 个月)和疾病进展(已证实或怀疑由 ECD 引起的病变的进展或恶化)。
结果 共确定了 63 名确诊为 ECD 的成年患者。他们诊断 ECD 时的中位年龄为 54 岁(年龄范围为 18-80 岁),67%(63 人中的 42 人)为男性。克拉屈滨是最常用的化疗药物,63 名患者中有 21 名(33%)使用了克拉屈滨。他们在接受克拉屈滨治疗时的中位年龄为 62 岁(年龄范围为 40-78 岁)。克拉屈滨在 9 名患者中用作一线治疗,在其余 12 名患者中用作后线治疗。克拉屈滨给药周期的中位数为 2.5(范围,1-6)。总体临床反应率为 52%(17 人中的 9 人)(6% [17 人中的 1 人] 完全反应和 46% [17 人中的 8 人] 部分反应),18%(17 人中的 3 人)疾病稳定和 30%(5 17) 进行性疾病。在对克拉屈滨治疗有反应的患者中,临床反应的中位持续时间为 9 个月(范围 6-129 个月),2 名患者持续反应。总体放射学缓解率为 54%(15 人中有 8 人)(全部为部分缓解),26%(15 人中有 4 人)疾病稳定,20%(15 人中有 2 人)疾病进展。治疗相关的不良反应包括 2 种感染性并发症(肺炎和中心线感染,均需要住院治疗)和 2 种血液学不良反应(4 级中性粒细胞减少症和血小板减少症,以及 3 级中性粒细胞减少症,均需要停止治疗)。
结论和相关性 克拉屈滨治疗 ECD 的临床疗效适中,可考虑作为无BRAF V600E 突变病例的治疗选择。它通常具有良好的耐受性,并可能导致持久的反应。

























 京公网安备 11010802027423号
京公网安备 11010802027423号