当前位置:
X-MOL 学术
›
Eur. Polym. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Increasing complexity in organopolymerization of multifunctional γ -butyrolactones
European Polymer Journal ( IF 6 ) Pub Date : 2017-10-01 , DOI: 10.1016/j.eurpolymj.2017.05.043 Jing Tang , Eugene Y.-X. Chen
European Polymer Journal ( IF 6 ) Pub Date : 2017-10-01 , DOI: 10.1016/j.eurpolymj.2017.05.043 Jing Tang , Eugene Y.-X. Chen
|
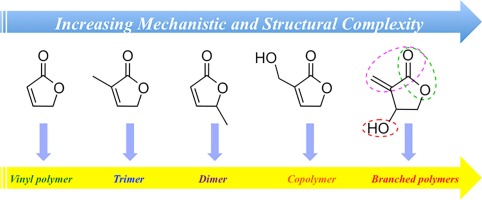
Abstract This contribution investigates organopolymerization of five multifunctional γ-butyrolactone-based monomers, including bifunctional (endocyclic double bond, lactone ring) dihydrofuran-2(3H)-one (FO), 3-methylfuran-2(5H)-one (3-MFO), and 5-methylfuran-2(5H)-one (5-MFO), as well as trifunctional (endocyclic or exocyclic double bond, lactone ring, hydroxyl group) 3-(hydroxymethyl) furan-2(5H)-one (3-HMFO) and β-hydroxy-α-methylene-γ-butyrolactone (βHMBL). The complexity of the reaction under nucleophilic and basic conditions using N-heterocyclic carbene (NHC) and superbase organic catalysts increases dramatically on going from the bifunctional monomers to the trifunctional ones. Thus, the polymerization of the parent FO leads to a vinyl-addition polymer, while the reaction of the base catalysts with the two methyl-substituted derivatives, 3-MFO and 5-MFO, affords predominately a trimer and dimer, respectively. The polymerization of trifunctional 3-HMFO gives a poly(vinyl–ether lactone) copolymer structure, via two different types of base activation mechanisms and a combination of Michael and ox-Michael additions and proton transfer processes. The polymerization of βHMBL has the highest degree of the complexity in this monomer series, due to its presence of both the reactive exocyclic double bond and hydroxyl group, producing a branched vinyl–ether lactone copolymer structure having six different types of substructural units. The results reveal multiple types of reaction pathways and their mechanistic crossovers involved in the βHMBL polymerization, including conjugate Michael and oxa-Michael additions and proton transfer processes, as well as ene-type dehydration reactions, enabled by proton transfer.
中文翻译:

增加多功能γ-丁内酯有机聚合的复杂性
摘要 本文研究了五种多功能 γ-丁内酯基单体的有机聚合反应,包括双功能(内环双键、内酯环)二氢呋喃-2(3H)-one (FO)、3-甲基呋喃-2(5H)-one (3- MFO) 和 5-甲基呋喃-2(5H)-one (5-MFO),以及三官能(环内或环外双键、内酯环、羟基)3-(羟甲基)呋喃-2(5H)-one (3-HMFO) 和 β-羟基-α-亚甲基-γ-丁内酯 (βHMBL)。在亲核和碱性条件下,使用 N-杂环卡宾 (NHC) 和超碱性有机催化剂的反应的复杂性随着从双官能单体到三官能单体而急剧增加。因此,母体 FO 的聚合产生乙烯基加成聚合物,而碱催化剂与两个甲基取代衍生物的反应,3-MFO 和 5-MFO 分别主要提供三聚体和二聚体。三官能 3-HMFO 的聚合通过两种不同类型的碱活化机制以及 Michael 和 ox-Michael 加成和质子转移过程的组合产生聚(乙烯基 - 醚内酯)共聚物结构。βHMBL 的聚合在该单体系列中具有最高程度的复杂性,因为它同时存在反应性环外双键和羟基,产生具有六种不同类型亚结构单元的支链乙烯基醚内酯共聚物结构。结果揭示了βHMBL聚合中涉及的多种反应途径及其机制交叉,包括共轭迈克尔和氧杂-迈克尔加成和质子转移过程,以及烯型脱水反应,
更新日期:2017-10-01
中文翻译:

增加多功能γ-丁内酯有机聚合的复杂性
摘要 本文研究了五种多功能 γ-丁内酯基单体的有机聚合反应,包括双功能(内环双键、内酯环)二氢呋喃-2(3H)-one (FO)、3-甲基呋喃-2(5H)-one (3- MFO) 和 5-甲基呋喃-2(5H)-one (5-MFO),以及三官能(环内或环外双键、内酯环、羟基)3-(羟甲基)呋喃-2(5H)-one (3-HMFO) 和 β-羟基-α-亚甲基-γ-丁内酯 (βHMBL)。在亲核和碱性条件下,使用 N-杂环卡宾 (NHC) 和超碱性有机催化剂的反应的复杂性随着从双官能单体到三官能单体而急剧增加。因此,母体 FO 的聚合产生乙烯基加成聚合物,而碱催化剂与两个甲基取代衍生物的反应,3-MFO 和 5-MFO 分别主要提供三聚体和二聚体。三官能 3-HMFO 的聚合通过两种不同类型的碱活化机制以及 Michael 和 ox-Michael 加成和质子转移过程的组合产生聚(乙烯基 - 醚内酯)共聚物结构。βHMBL 的聚合在该单体系列中具有最高程度的复杂性,因为它同时存在反应性环外双键和羟基,产生具有六种不同类型亚结构单元的支链乙烯基醚内酯共聚物结构。结果揭示了βHMBL聚合中涉及的多种反应途径及其机制交叉,包括共轭迈克尔和氧杂-迈克尔加成和质子转移过程,以及烯型脱水反应,


























 京公网安备 11010802027423号
京公网安备 11010802027423号