PLoS Pathogens ( IF 6.7 ) Pub Date : 2017-08-30 , DOI: 10.1371/journal.ppat.1006596 Alessandra De Leo , Horng-Shen Chen , Chih-Chi Andrew Hu , Paul M. Lieberman
|
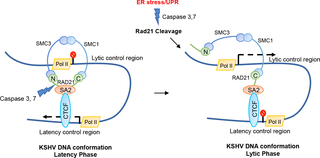
Kaposi’s sarcoma (KS)-associated herpesvirus (KSHV) is a human gammaherpesvirus recognized as the principal causative agent of KS and primary effusion lymphoma (PEL). KSHV establishes persistent latent infection in B lymphocytes where viral gene expression is restricted, in part, by a cohesin-dependent chromosome conformation. Here, we show that endoplasmic reticulum (ER) stress induces a rapid, caspase-dependent cleavage of cohesin subunit RAD21. ER stress-induced cleavage of RAD21 correlated with a rapid and strong viral lytic transcriptional activation. This effect was observed in several KSHV positive PEL cells, but not in other B-cells or non-B-cell models of KSHV latency. The cleaved-RAD21 does not dissociate from viral genomes, nor disassemble from other components of the cohesin complex. However, RAD21 cleavage correlated with the disruption of the latency genome conformation as revealed by chromosome conformation capture (3C). Ectopic expression of C-terminal RAD21 cleaved form could partially induce KSHV lytic genes transcription in BCBLI cells, suggesting that ER-stress induced RAD21 cleavage was sufficient to induce KSHV reactivation from latency in PEL cells. Taken together our results reveal a novel aspect for control and maintenance of KSHV genome latency conformation mediated by stress-induced RAD21 cleavage. Our studies also suggest that RAD21 cleavage may be a general regulatory mechanism for rapid alteration of cellular chromosome conformation and cohesin-dependent transcription regulation.
中文翻译:

内质网应激和胱天蛋白酶依赖的RAD21裂解解除KSHV潜伏构象。
卡波西氏肉瘤(KS)相关的疱疹病毒(KSHV)是一种人类γ疱疹病毒,被认为是KS和原发渗出性淋巴瘤(PEL)的主要病原体。KSHV在B淋巴细胞中建立持续的潜伏感染,其中病毒基因的表达部分受黏附素依赖性染色体构象的限制。在这里,我们表明内质网(ER)应力诱导cohesin亚基RAD21的快速,caspase依赖裂解。内质网应激诱导的RAD21裂解与快速和强大的病毒裂解转录激活相关。在几个KSHV阳性PEL细胞中观察到了这种效应,但在其他B细胞或KSHV潜伏期的非B细胞模型中则未观察到这种效应。切割的RAD21不从病毒基因组解离,也不从粘着蛋白复合物的其他组分分解。然而,RAD21裂解与潜伏基因组构象的破坏有关,如染色体构象捕获(3C)所揭示。C末端RAD21切割形式的异位表达可以部分诱导BCBLI细胞中的KSHV裂解基因转录,表明ER应激诱导的RAD21切割足以诱导PEL细胞中潜伏期的KSHV重新激活。总之,我们的结果揭示了由应激诱导的RAD21切割介导的控制和维持KSHV基因组潜伏性构象的新方面。我们的研究还表明,RAD21裂解可能是细胞染色体构象快速改变和黏附素依赖性转录调控的一般调控机制。C末端RAD21切割形式的异位表达可以部分诱导BCBLI细胞中的KSHV裂解基因转录,表明ER应激诱导的RAD21切割足以诱导PEL细胞中潜伏期的KSHV重新激活。总之,我们的结果揭示了由应激诱导的RAD21切割介导的控制和维持KSHV基因组潜伏性构象的新方面。我们的研究还表明,RAD21裂解可能是细胞染色体构象快速改变和黏附素依赖性转录调控的一般调控机制。C末端RAD21裂解形式的异位表达可以部分诱导BCBLI细胞中KSHV裂解基因的转录,这表明内质网应激诱导的RAD21裂解足以诱导PEL细胞潜伏期的KSHV激活。总之,我们的结果揭示了由应激诱导的RAD21切割介导的控制和维持KSHV基因组潜伏性构象的新方面。我们的研究还表明,RAD21裂解可能是细胞染色体构象快速改变和黏附素依赖性转录调控的一般调控机制。总之,我们的结果揭示了由应激诱导的RAD21切割介导的控制和维持KSHV基因组潜伏性构象的新方面。我们的研究还表明,RAD21裂解可能是细胞染色体构象快速改变和黏附素依赖性转录调控的一般调控机制。总之,我们的结果揭示了由应激诱导的RAD21切割介导的控制和维持KSHV基因组潜伏性构象的新方面。我们的研究还表明,RAD21裂解可能是细胞染色体构象快速改变和黏附素依赖性转录调控的一般调控机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号