PLoS Pathogens ( IF 6.7 ) Pub Date : 2017-09-07 , DOI: 10.1371/journal.ppat.1006557 Angélique Igel-Egalon , Mohammed Moudjou , Davy Martin , Alexandra Busley , Tina Knäpple , Laetitia Herzog , Fabienne Reine , Nad’a Lepejova , Charles-Adrien Richard , Vincent Béringue , Human Rezaei
|
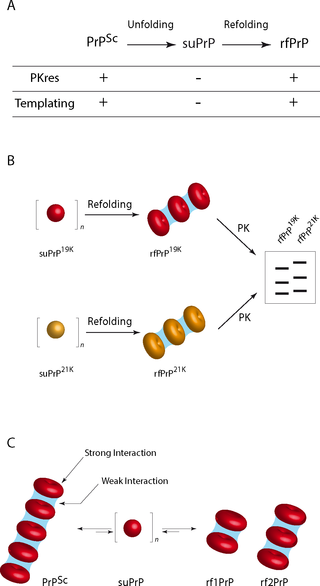
Mammalian prions, the pathogens that cause transmissible spongiform encephalopathies, propagate by self-perpetuating the structural information stored in the abnormally folded, aggregated conformer (PrPSc) of the host-encoded prion protein (PrPC). To date, no structural model related to prion assembly organization satisfactorily describes how strain-specified structural information is encoded and by which mechanism this information is transferred to PrPC. To achieve progress on this issue, we correlated the PrPSc quaternary structural transition from three distinct prion strains during unfolding and refolding with their templating activity. We reveal the existence of a mesoscopic organization in PrPSc through the packing of a highly stable oligomeric elementary subunit (suPrP), in which the strain structural determinant (SSD) is encoded. Once kinetically trapped, this elementary subunit reversibly loses all replicative information. We demonstrate that acquisition of the templating interface and infectivity requires structural rearrangement of suPrP, in concert with its condensation. The existence of such an elementary brick scales down the SSD support to a small oligomer and provide a basis of reflexion for prion templating process and propagation.
中文翻译:

传染性ion病毒组装体的可逆展开揭示了低聚基本砖的存在
哺乳动物病毒是引起可传播的海绵状脑病的病原体,它们通过自我保存存储在宿主编码的ion病毒蛋白(PrP C)的异常折叠的聚集构象异构体(PrP Sc)中的结构信息自我繁殖。迄今为止,有关朊病毒组件组织没有结构模型令人满意地描述了应变指定的结构信息的编码方式和通过该机构这个信息被转移到的PrP Ç。为了在这个问题上取得进展,我们将三个不同的病毒菌株在展开和重新折叠过程中的PrP Sc季结构转变与它们的模板活性相关联。我们揭示了PrP Sc中存在介观组织通过包装高度稳定的寡聚基本亚单位(suPrP),其中编码了应变结构决定簇(SSD)。一旦被动力学捕获,该基本亚基可逆地丢失所有复制信息。我们证明模板界面和传染性的获取需要suPrP的结构重排,以及其缩合。这种基本砖的存在将SSD的支撑缩减为小的低聚物,并为病毒模板化过程和传播提供了反射的基础。


























 京公网安备 11010802027423号
京公网安备 11010802027423号