当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights on a Metal‐Free Borylative Cyclization of Alkynes Using BCl3: A Theoretical Investigation
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-08-29 , DOI: 10.1002/ajoc.201700343 Yu Wei 1 , Dongwei Liu 1 , Xinlin Qing 1 , Liang Xu 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2017-08-29 , DOI: 10.1002/ajoc.201700343 Yu Wei 1 , Dongwei Liu 1 , Xinlin Qing 1 , Liang Xu 1
Affiliation
|
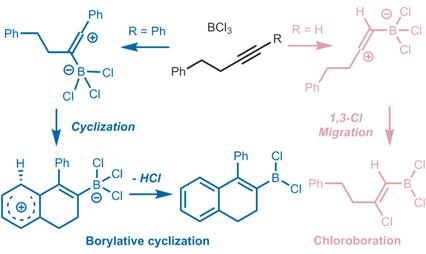
The metal‐free reactions between alkynes and BCl3 were investigated by means of DFT calculations, focusing on elucidating the detailed cyclization mechanism and the different chemoselectivity between internal and terminal alkynes. The results showed that borylative cyclization of internal alkynes proceeded via kinetically favorable stepwise complexation, electrophilic dearomatization, hydrogen migration and rearomatization. In contrast, for terminal alkynes, the competitive chloroboration step, which followed the complexation step, was more feasible than electrophilic dearomatization, affording the thermodynamically stable addition products irreversibly.
中文翻译:

使用BCl3的炔烃无金属硼化环化机理的理论研究:理论研究
通过DFT计算研究了炔烃与BCl 3之间的无金属反应,重点在于阐明详细的环化机理以及内部和末端炔烃之间的不同化学选择性。结果表明,内部炔烃的硼化环化反应是通过动力学上有利的逐步络合,亲电脱芳香化作用,氢迁移和重金属化进行的。相反,对于末端炔烃,竞争性的氯硼化步骤(在络合步骤之后)比亲电脱芳香化步骤更可行,从而不可逆地提供了热力学稳定的加成产物。
更新日期:2017-08-29
中文翻译:

使用BCl3的炔烃无金属硼化环化机理的理论研究:理论研究
通过DFT计算研究了炔烃与BCl 3之间的无金属反应,重点在于阐明详细的环化机理以及内部和末端炔烃之间的不同化学选择性。结果表明,内部炔烃的硼化环化反应是通过动力学上有利的逐步络合,亲电脱芳香化作用,氢迁移和重金属化进行的。相反,对于末端炔烃,竞争性的氯硼化步骤(在络合步骤之后)比亲电脱芳香化步骤更可行,从而不可逆地提供了热力学稳定的加成产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号