当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cleaning of pH Selective Electrodes with Ionophore-Doped Fluorous Membranes in NaOH Solution at 90 °C
Electroanalysis ( IF 3 ) Pub Date : 2017-07-20 , DOI: 10.1002/elan.201700228 Elizabeth C. Lugert‐Thom 1 , John A. Gladysz 2 , József Rábai 3 , Philippe Bühlmann 1
Electroanalysis ( IF 3 ) Pub Date : 2017-07-20 , DOI: 10.1002/elan.201700228 Elizabeth C. Lugert‐Thom 1 , John A. Gladysz 2 , József Rábai 3 , Philippe Bühlmann 1
Affiliation
|
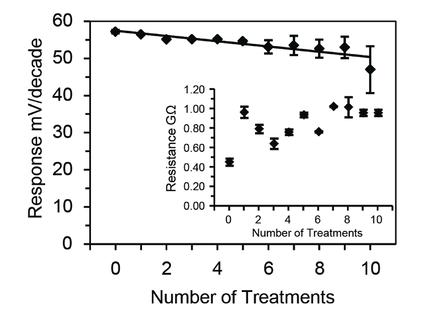
This work demonstrates the remarkable stability of fluorous ion-selective electrode (ISE) membranes by exposing them to cleaning-in-place treatments (CIP) as they are used in many industrial processes. The sensing membranes consisted of Teflon AF2400 plasticized with a linear perfluoropolyether and doped with ionic sites and a H+ ionophore (i. e., tris[3-(perfluorooctyl)propyl]amine, 1, or tris[3-(perfluorooctyl)pentyl]amine, 2). To mimic a typical CIP treatment, the electrodes were repeatedly exposed for 30 min to a 3.0 % NaOH solution at 90 °C (pH 12.7). ISE membranes doped with the less strongly H+ binding ionophore 1 started to show reduced potentiometric response slopes and increased resistances after one exposure for 30 min to hot 3.0 % NaOH solution. No decomposition of the ionic sites and ionophore 1 at 90 °C was evident by 1H NMR spectroscopy, suggesting that the performance of membranes doped with 1 was compromised primarily by leaching of the negatively charged ionic sites along with H+ into the hot caustic solution. In contrast, even after ten exposures to hot 3.0 % NaOH for a cumulative 5 h at 90 °C, the fluorous sensing membranes doped with the more strongly H+ binding ionophore 2 still showed the ability to respond with a theoretical (Nernstian) slope up to pH 12. Addition of the fluorophilic electrolyte salt methyltris[3-(perfluorooctyl)propyl]ammonium tetrakis[3, 5-bis(perfluorohexyl)phenyl]borate reduced the membrane resistance by an order of magnitude.
中文翻译:

在 90 °C 的 NaOH 溶液中用掺杂离子载体的氟膜清洗 pH 选择电极
这项工作通过将氟离子选择性电极 (ISE) 膜暴露于原位清洗处理 (CIP) 来证明其显着的稳定性,因为它们在许多工业过程中使用。传感膜由用线性全氟聚醚增塑并掺杂离子位点和 H+ 离子载体(即三[3-(全氟辛基)丙基]胺,1 或三[3-(全氟辛基)戊基]胺,2)的 Teflon AF2400 组成)。为了模拟典型的 CIP 处理,将电极反复暴露于 90 °C (pH 12.7) 的 3.0% NaOH 溶液中 30 分钟。在与热的 3.0% NaOH 溶液接触 30 分钟后,掺杂有较弱的 H+ 结合离子载体 1 的 ISE 膜开始显示出降低的电位响应斜率和增加的电阻。1H NMR 光谱表明,离子位点和离子载体 1 在 90 °C 下没有分解,这表明掺杂 1 的膜的性能主要由于带负电的离子位点与 H+ 一起浸入热苛性碱溶液中而受到损害。相比之下,即使在 90 °C 下 10 次暴露于 3.0% NaOH 累积 5 小时后,掺杂有更强的 H+ 结合离子载体 2 的氟传感膜仍显示出响应理论(能斯脱)斜率的能力pH 12。添加四[3, 5-双(全氟己基)苯基]硼酸甲基三[3-(全氟辛基)丙基]铵的亲氟电解质盐使膜电阻降低一个数量级。表明掺杂 1 的膜的性能主要受到带负电荷的离子位点与 H+ 一起浸入热苛性碱溶液的影响。相比之下,即使在 90 °C 下 10 次暴露于 3.0% NaOH 累积 5 小时后,掺杂有更强的 H+ 结合离子载体 2 的氟传感膜仍显示出响应理论(能斯脱)斜率的能力pH 12。添加四[3, 5-双(全氟己基)苯基]硼酸甲基三[3-(全氟辛基)丙基]铵的亲氟电解质盐使膜电阻降低一个数量级。表明掺杂 1 的膜的性能主要受到带负电荷的离子位点与 H+ 一起浸入热苛性碱溶液的影响。相比之下,即使在 90 °C 下 10 次暴露于 3.0% NaOH 累积 5 小时后,掺杂有更强的 H+ 结合离子载体 2 的氟传感膜仍显示出响应理论(能斯脱)斜率的能力pH 12。添加四[3, 5-双(全氟己基)苯基]硼酸甲基三[3-(全氟辛基)丙基]铵的亲氟电解质盐使膜电阻降低一个数量级。
更新日期:2017-07-20
中文翻译:

在 90 °C 的 NaOH 溶液中用掺杂离子载体的氟膜清洗 pH 选择电极
这项工作通过将氟离子选择性电极 (ISE) 膜暴露于原位清洗处理 (CIP) 来证明其显着的稳定性,因为它们在许多工业过程中使用。传感膜由用线性全氟聚醚增塑并掺杂离子位点和 H+ 离子载体(即三[3-(全氟辛基)丙基]胺,1 或三[3-(全氟辛基)戊基]胺,2)的 Teflon AF2400 组成)。为了模拟典型的 CIP 处理,将电极反复暴露于 90 °C (pH 12.7) 的 3.0% NaOH 溶液中 30 分钟。在与热的 3.0% NaOH 溶液接触 30 分钟后,掺杂有较弱的 H+ 结合离子载体 1 的 ISE 膜开始显示出降低的电位响应斜率和增加的电阻。1H NMR 光谱表明,离子位点和离子载体 1 在 90 °C 下没有分解,这表明掺杂 1 的膜的性能主要由于带负电的离子位点与 H+ 一起浸入热苛性碱溶液中而受到损害。相比之下,即使在 90 °C 下 10 次暴露于 3.0% NaOH 累积 5 小时后,掺杂有更强的 H+ 结合离子载体 2 的氟传感膜仍显示出响应理论(能斯脱)斜率的能力pH 12。添加四[3, 5-双(全氟己基)苯基]硼酸甲基三[3-(全氟辛基)丙基]铵的亲氟电解质盐使膜电阻降低一个数量级。表明掺杂 1 的膜的性能主要受到带负电荷的离子位点与 H+ 一起浸入热苛性碱溶液的影响。相比之下,即使在 90 °C 下 10 次暴露于 3.0% NaOH 累积 5 小时后,掺杂有更强的 H+ 结合离子载体 2 的氟传感膜仍显示出响应理论(能斯脱)斜率的能力pH 12。添加四[3, 5-双(全氟己基)苯基]硼酸甲基三[3-(全氟辛基)丙基]铵的亲氟电解质盐使膜电阻降低一个数量级。表明掺杂 1 的膜的性能主要受到带负电荷的离子位点与 H+ 一起浸入热苛性碱溶液的影响。相比之下,即使在 90 °C 下 10 次暴露于 3.0% NaOH 累积 5 小时后,掺杂有更强的 H+ 结合离子载体 2 的氟传感膜仍显示出响应理论(能斯脱)斜率的能力pH 12。添加四[3, 5-双(全氟己基)苯基]硼酸甲基三[3-(全氟辛基)丙基]铵的亲氟电解质盐使膜电阻降低一个数量级。


























 京公网安备 11010802027423号
京公网安备 11010802027423号