Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Understanding of Electrochemical Mechanisms for CO2 Capture and Conversion into Hydrocarbon Fuels in Transition-Metal Carbides (MXenes)
ACS Nano ( IF 17.1 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acsnano.7b03738 Neng Li 1, 2 , Xingzhu Chen 1 , Wee-Jun Ong 3 , Douglas R. MacFarlane 4 , Xiujian Zhao 1 , Anthony K. Cheetham 2 , Chenghua Sun 5
ACS Nano ( IF 17.1 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acsnano.7b03738 Neng Li 1, 2 , Xingzhu Chen 1 , Wee-Jun Ong 3 , Douglas R. MacFarlane 4 , Xiujian Zhao 1 , Anthony K. Cheetham 2 , Chenghua Sun 5
Affiliation
|
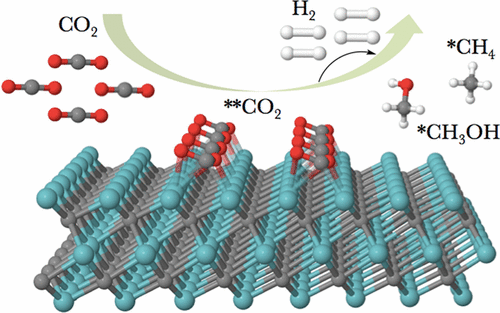
Two-dimensional (2D) transition-metal (groups IV, V, VI) carbides (MXenes) with formulas M3C2 have been investigated as CO2 conversion catalysts with well-resolved density functional theory calculations. While MXenes from the group IV to VI series have demonstrated an active behavior for the capture of CO2, the Cr3C2 and Mo3C2 MXenes exhibit the most promising CO2 to CH4 selective conversion capabilities. Our results predicted the formation of OCHO• and HOCO• radical species in the early hydrogenation steps through spontaneous reactions. This provides atomic level insights into the computer-aided screening for high-performance catalysts and the understanding of electrochemical mechanisms for CO2 reduction to energy-rich hydrocarbon fuels, which is of fundamental significance to elucidate the elementary steps for CO2 fixation.
中文翻译:

了解过渡金属碳化物(MXenes)中CO 2捕集并转化为碳氢化合物燃料的电化学机理
分子式为M 3 C 2的二维(2D)过渡金属(IV,V,VI族)碳化物(MXenes)已作为CO 2转化催化剂进行了研究,具有很好的密度泛函理论计算能力。虽然IV至VI系列的MXene已表现出捕获CO 2的活跃行为,但Cr 3 C 2和Mo 3 C 2 MXene表现出最有前途的CO 2至CH 4选择性转化能力。我们的结果预测了OCHO •和HOCO •的形成早期氢化中的自由基种类通过自发反应进行。这为计算机辅助筛选高性能催化剂提供了原子级的见解,并为将CO 2还原为富能烃燃料提供了电化学机理的理解,这对于阐明CO 2固定的基本步骤具有根本意义。
更新日期:2017-09-13
中文翻译:

了解过渡金属碳化物(MXenes)中CO 2捕集并转化为碳氢化合物燃料的电化学机理
分子式为M 3 C 2的二维(2D)过渡金属(IV,V,VI族)碳化物(MXenes)已作为CO 2转化催化剂进行了研究,具有很好的密度泛函理论计算能力。虽然IV至VI系列的MXene已表现出捕获CO 2的活跃行为,但Cr 3 C 2和Mo 3 C 2 MXene表现出最有前途的CO 2至CH 4选择性转化能力。我们的结果预测了OCHO •和HOCO •的形成早期氢化中的自由基种类通过自发反应进行。这为计算机辅助筛选高性能催化剂提供了原子级的见解,并为将CO 2还原为富能烃燃料提供了电化学机理的理解,这对于阐明CO 2固定的基本步骤具有根本意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号