当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Collagen Fibril Intermolecular Spacing Changes with 2-Propanol: A Mechanism for Tissue Stiffness
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00418 Hannah C. Wells 1 , Katie H. Sizeland 1, 2 , Susyn J.R. Kelly 1 , Nigel Kirby 2 , Adrian Hawley 2 , Stephen Mudie 2 , Richard G. Haverkamp 1
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2017-09-13 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00418 Hannah C. Wells 1 , Katie H. Sizeland 1, 2 , Susyn J.R. Kelly 1 , Nigel Kirby 2 , Adrian Hawley 2 , Stephen Mudie 2 , Richard G. Haverkamp 1
Affiliation
|
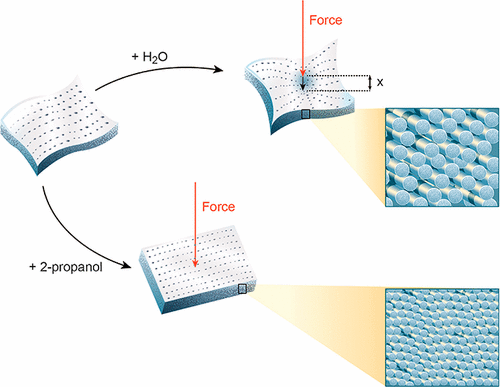
Materials composed primarily of collagen are important as surgical scaffolds and other medical devices and require flexibility. However, the factors that control the suppleness and flexibility of these materials are not well understood. Acellular dermal matrix materials in aqueous mixtures of 2-propanol were studied. Synchrotron-based small-angle X-ray scattering was used to characterize the collagen structure and structural arrangement. Stiffness was measured by bend tests. Bend modulus increased logarithmically with 2-propanol concentration from 0.5 kPa in water to 103 kPa in pure 2-propanol. The intermolecular spacing between tropocollagen molecules decreased from 15.3 to 11.4 Å with increasing 2-propanol concentration while fibril diameter decreased from 57.2 to 37.2 nm. D-spacing initially increased from 63.6 to 64.2 nm at 50% 2-propanol then decreased to 60.3 nm in pure 2-propanol. The decrease in intermolecular spacing and fibril diameter are due to removal of water and the collapse of the hydrogen bond structure between tropocollagen molecules causing closer packing of the molecules within a fibril. We speculate this tighter molecular packing may restrict the sliding of collagen within fibrils, and similar disruption of the extended hydration layer between fibrils may lead to restriction of sliding between fibrils. This mechanism for tissue stiffness may be more general.
中文翻译:

2-丙醇的胶原原纤维分子间间距变化:组织刚度的机制。
主要由胶原蛋白组成的材料对于外科手术支架和其他医疗设备很重要,并且需要柔韧性。但是,控制这些材料的柔软性和柔韧性的因素还不是很清楚。研究了2-丙醇水性混合物中的无细胞真皮基质材料。基于同步加速器的小角度X射线散射用于表征胶原蛋白的结构和结构排列。刚度通过弯曲试验测量。当2-丙醇浓度从水中的0.5 kPa增加到纯2-丙醇中的103 kPa时,弯曲模量呈对数增加。随着2-丙醇浓度的增加,对流胶原蛋白分子之间的分子间间距从15.3减小到11.4,而原纤维直径从57.2 nm减小到37.2 nm。D间距最初从63.6增加到64。在50%2-丙醇中为2 nm,然后在纯2-丙醇中降至60.3 nm。分子间间距和原纤维直径的减小是由于水分的除去和原胶原蛋白分子之间氢键结构的崩溃,导致分子在原纤维内的紧密堆积。我们推测这种更紧密的分子堆积可能会限制胶原蛋白在原纤维内的滑动,并且类似的破坏原纤维之间延伸的水合层可能会导致原纤维之间的滑动受到限制。这种组织刚度的机制可能更普遍。我们推测这种更紧密的分子堆积可能会限制胶原蛋白在原纤维内的滑动,并且类似的破坏原纤维之间延伸的水合层可能会导致原纤维之间的滑动受到限制。这种组织刚度的机制可能更普遍。我们推测这种更紧密的分子堆积可能会限制胶原蛋白在原纤维内的滑动,并且类似的破坏原纤维之间延伸的水合层可能会导致原纤维之间滑动的限制。这种组织刚度的机制可能更普遍。
更新日期:2017-09-13
中文翻译:

2-丙醇的胶原原纤维分子间间距变化:组织刚度的机制。
主要由胶原蛋白组成的材料对于外科手术支架和其他医疗设备很重要,并且需要柔韧性。但是,控制这些材料的柔软性和柔韧性的因素还不是很清楚。研究了2-丙醇水性混合物中的无细胞真皮基质材料。基于同步加速器的小角度X射线散射用于表征胶原蛋白的结构和结构排列。刚度通过弯曲试验测量。当2-丙醇浓度从水中的0.5 kPa增加到纯2-丙醇中的103 kPa时,弯曲模量呈对数增加。随着2-丙醇浓度的增加,对流胶原蛋白分子之间的分子间间距从15.3减小到11.4,而原纤维直径从57.2 nm减小到37.2 nm。D间距最初从63.6增加到64。在50%2-丙醇中为2 nm,然后在纯2-丙醇中降至60.3 nm。分子间间距和原纤维直径的减小是由于水分的除去和原胶原蛋白分子之间氢键结构的崩溃,导致分子在原纤维内的紧密堆积。我们推测这种更紧密的分子堆积可能会限制胶原蛋白在原纤维内的滑动,并且类似的破坏原纤维之间延伸的水合层可能会导致原纤维之间的滑动受到限制。这种组织刚度的机制可能更普遍。我们推测这种更紧密的分子堆积可能会限制胶原蛋白在原纤维内的滑动,并且类似的破坏原纤维之间延伸的水合层可能会导致原纤维之间的滑动受到限制。这种组织刚度的机制可能更普遍。我们推测这种更紧密的分子堆积可能会限制胶原蛋白在原纤维内的滑动,并且类似的破坏原纤维之间延伸的水合层可能会导致原纤维之间滑动的限制。这种组织刚度的机制可能更普遍。


























 京公网安备 11010802027423号
京公网安备 11010802027423号