当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Situ Spectroscopy and Mechanistic Insights into CO Oxidation on Transition-Metal-Substituted Ceria Nanoparticles
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acscatal.7b01600 Joseph S. Elias , Kelsey A. Stoerzinger , Wesley T. Hong , Marcel Risch , Livia Giordano 1 , Azzam N. Mansour 2 , Yang Shao-Horn
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acscatal.7b01600 Joseph S. Elias , Kelsey A. Stoerzinger , Wesley T. Hong , Marcel Risch , Livia Giordano 1 , Azzam N. Mansour 2 , Yang Shao-Horn
Affiliation
|
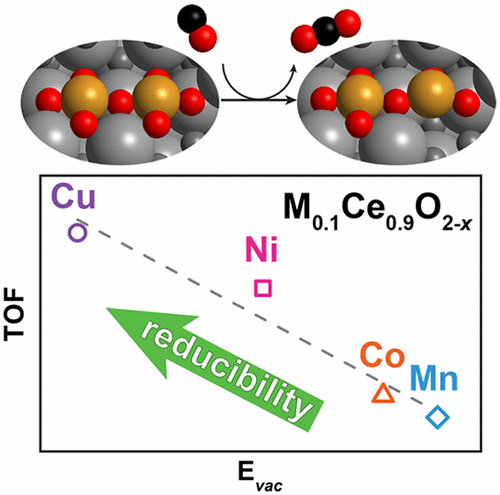
Herein we investigate the reaction intermediates formed during CO oxidation on copper-substituted ceria nanoparticles (Cu0.1Ce0.9O2–x) by means of in situ spectroscopic techniques and identify an activity descriptor that rationalizes a trend with other metal substitutes (M0.1Ce0.9O2–x, M = Mn, Fe, Co, Ni). In situ X-ray absorption spectroscopy (XAS) performed under catalytic conditions demonstrates that O2– transfer occurs at dispersed copper centers, which are redox active during catalysis. In situ XAS reveals a dramatic reduction at the copper centers that is fully reversible under catalytic conditions, which rationalizes the high catalytic activity of Cu0.1Ce0.9O2–x. Ambient pressure X-ray photoelectron spectroscopy (AP-XPS) and in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) show that CO can be oxidized to CO32– in the absence of O2. We find that CO32– desorbs as CO2 only under oxygen-rich conditions when the oxygen vacancy is filled by the dissociative adsorption of O2. These data, along with kinetic analyses, lend support to a mechanism in which the breaking of copper–oxygen bonds is rate-determining under oxygen-rich conditions, while refilling the resulting oxygen vacancy is rate-determining under oxygen-lean conditions. On the basis of these observations and density functional calculations, we introduce the computed oxygen vacancy formation energy (Evac) as an activity descriptor for substituted ceria materials and demonstrate that Evac successfully rationalizes the trend in the activities of M0.1Ce0.9O2–x catalysts that spans three orders of magnitude. The applicability of Evac as a useful design descriptor is demonstrated by the catalytic performance of the ternary oxide Cu0.1La0.1Ce0.8O2–x, which has an apparent activation energy rivaling those of state-of-the-art Au/TiO2 materials. Thus, we suggest that cost-effective catalysts for CO oxidation can be rationally designed by judicious choice of substituting metal through the computational screening of Evac.
中文翻译:

过渡金属取代的二氧化铈纳米粒子的原位光谱和机理研究
本文中,我们通过原位光谱技术研究了在CO氧化过程中在铜取代的二氧化铈纳米颗粒(Cu 0.1 Ce 0.9 O 2– x)上形成的反应中间体,并确定了与其他金属替代物(M 0.1 Ce 0.9 O 2– x,M = Mn,Fe,Co,Ni)。在催化条件下进行的原位X射线吸收光谱(XAS)表明,O 2–转移发生在分散的铜中心,这些铜在催化过程中具有氧化还原活性。原位XAS分析表明,在催化条件下铜中心的还原是完全可逆的,这使Cu 0.1 Ce 0.9 O 2– x的高催化活性合理化。环境压力X射线光电子能谱法(AP-XPS)和原位漫反射红外线傅里叶变换光谱法(DRIFTS)表明,CO可被氧化成CO 3 2-不存在的O- 2。我们发现,CO 3 2-脱附的CO 2仅在富氧条件下,当氧空位是通过的O-解离吸附填充2。这些数据以及动力学分析为以下机制提供了支持:在富氧条件下,决定铜-氧键的断裂速率是决定性的,而在贫氧条件下,重新填充所产生的氧空位则是决定速率的机理。基于这些观察和密度泛函计算,我们引入了计算出的氧空位形成能(E vac)作为取代二氧化铈材料的活性描述子,并证明E vac成功地使M 0.1 Ce 0.9 O 2的活性趋势合理化。– x跨越三个数量级的催化剂。E vac的适用性三元氧化物Cu 0.1 La 0.1 Ce 0.8 O 2– x的催化性能证明了其为有用的设计描述符,该催化剂的表观活化能可与最新的Au / TiO 2材料媲美。因此,我们建议可以通过E vac的计算筛选,通过明智地选择取代金属来合理设计具有成本效益的CO氧化催化剂。
更新日期:2017-09-12
中文翻译:

过渡金属取代的二氧化铈纳米粒子的原位光谱和机理研究
本文中,我们通过原位光谱技术研究了在CO氧化过程中在铜取代的二氧化铈纳米颗粒(Cu 0.1 Ce 0.9 O 2– x)上形成的反应中间体,并确定了与其他金属替代物(M 0.1 Ce 0.9 O 2– x,M = Mn,Fe,Co,Ni)。在催化条件下进行的原位X射线吸收光谱(XAS)表明,O 2–转移发生在分散的铜中心,这些铜在催化过程中具有氧化还原活性。原位XAS分析表明,在催化条件下铜中心的还原是完全可逆的,这使Cu 0.1 Ce 0.9 O 2– x的高催化活性合理化。环境压力X射线光电子能谱法(AP-XPS)和原位漫反射红外线傅里叶变换光谱法(DRIFTS)表明,CO可被氧化成CO 3 2-不存在的O- 2。我们发现,CO 3 2-脱附的CO 2仅在富氧条件下,当氧空位是通过的O-解离吸附填充2。这些数据以及动力学分析为以下机制提供了支持:在富氧条件下,决定铜-氧键的断裂速率是决定性的,而在贫氧条件下,重新填充所产生的氧空位则是决定速率的机理。基于这些观察和密度泛函计算,我们引入了计算出的氧空位形成能(E vac)作为取代二氧化铈材料的活性描述子,并证明E vac成功地使M 0.1 Ce 0.9 O 2的活性趋势合理化。– x跨越三个数量级的催化剂。E vac的适用性三元氧化物Cu 0.1 La 0.1 Ce 0.8 O 2– x的催化性能证明了其为有用的设计描述符,该催化剂的表观活化能可与最新的Au / TiO 2材料媲美。因此,我们建议可以通过E vac的计算筛选,通过明智地选择取代金属来合理设计具有成本效益的CO氧化催化剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号