当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparative Study of REDOR and CPPI Derived Order Parameters by 1H-Detected MAS NMR and MD Simulations
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.jpcb.7b06812 Sam Asami 1 , Bernd Reif 1, 2
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.jpcb.7b06812 Sam Asami 1 , Bernd Reif 1, 2
Affiliation
|
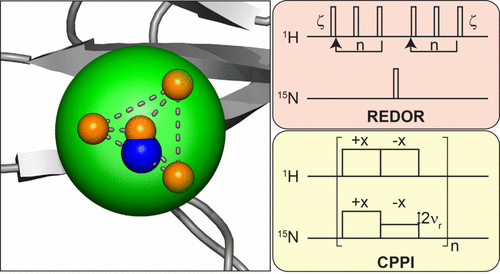
The measurement of dipolar couplings among directly bonded nuclei yields direct information on the amplitude of dynamic processes in the solid-state. For a reliable motional analysis using, e.g., the model-free approach, a correct quantification of the absolute values of these order parameters is absolutely essential. In the absence of a reference value for the rigid limit, too low dipolar coupling values might be misinterpreted as motion. Therefore, a detailed understanding of the effects that influence the quantification of the experimental order parameters is necessary. We compare here REDOR and CPPI derived order parameters assessed in 1H-detected experiments, and discuss the influence of remote protons and rf inhomogeneity on the extracted dipolar coupling constant for MAS rotation frequencies in the range 20–100 kHz. Experimental results are furthermore compared with the order parameter obtained from a molecular dynamics simulation. We find that fast magic-angle spinning up to 100 kHz can yield artifact-free REDOR based 1H,15N order parameters for perdeuterated and 100% amide back-exchanged proteins, and potentially even in uniformly protonated samples. We believe that awareness of systematic errors introduced by the measurement and in the analysis of order parameters will yield a better understanding of the dynamic properties of a protein derived from solid-state NMR observables.
中文翻译:

通过1 H检测的MAS NMR和MD模拟对REDOR和CPPI衍生阶参数的比较研究
对直接键合原子核之间偶极耦合的测量可得出有关固态动力学过程振幅的直接信息。为了使用例如无模型方法进行可靠的运动分析,绝对必须对这些阶次参数的绝对值进行正确的量化。在没有用于刚性极限的参考值的情况下,太低的偶极耦合值可能会被误解为运动。因此,有必要详细了解影响实验顺序参数量化的影响。我们在这里比较REDOR和CPPI得出的订单参数评估为1H检测的实验,并讨论了在20-100 kHz范围内的MAS旋转频率下,远程质子和rf不均匀性对提取的偶极耦合常数的影响。此外,将实验结果与从分子动力学模拟获得的有序参数进行了比较。我们发现,高达100 kHz的快速魔术角旋转可以产生基于无氘REDOR的1 H,15 N阶参数,用于氘化和100%酰胺反向交换的蛋白质,甚至可能在均匀质子化的样品中也是如此。我们相信,对通过测量和在阶数参数分析中引入的系统误差的认识将使人们更好地理解从固态NMR可观察到的蛋白质的动态特性。
更新日期:2017-09-12
中文翻译:

通过1 H检测的MAS NMR和MD模拟对REDOR和CPPI衍生阶参数的比较研究
对直接键合原子核之间偶极耦合的测量可得出有关固态动力学过程振幅的直接信息。为了使用例如无模型方法进行可靠的运动分析,绝对必须对这些阶次参数的绝对值进行正确的量化。在没有用于刚性极限的参考值的情况下,太低的偶极耦合值可能会被误解为运动。因此,有必要详细了解影响实验顺序参数量化的影响。我们在这里比较REDOR和CPPI得出的订单参数评估为1H检测的实验,并讨论了在20-100 kHz范围内的MAS旋转频率下,远程质子和rf不均匀性对提取的偶极耦合常数的影响。此外,将实验结果与从分子动力学模拟获得的有序参数进行了比较。我们发现,高达100 kHz的快速魔术角旋转可以产生基于无氘REDOR的1 H,15 N阶参数,用于氘化和100%酰胺反向交换的蛋白质,甚至可能在均匀质子化的样品中也是如此。我们相信,对通过测量和在阶数参数分析中引入的系统误差的认识将使人们更好地理解从固态NMR可观察到的蛋白质的动态特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号