当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Charge Transfer Complexes Formed by Heterocyclic Thioamides and Tetracyanoethylene: Experimental and Theoretical Study
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.jpca.7b00564 Tatiana S. Kolesnikova 1 , Margarita S. Chernov’yants 1 , Mikhail E. Kletskii 1 , Oleg N. Burov 1 , Gennady I. Bondarenko 1 , Pavel A. Knyazev 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-12 00:00:00 , DOI: 10.1021/acs.jpca.7b00564 Tatiana S. Kolesnikova 1 , Margarita S. Chernov’yants 1 , Mikhail E. Kletskii 1 , Oleg N. Burov 1 , Gennady I. Bondarenko 1 , Pavel A. Knyazev 2
Affiliation
|
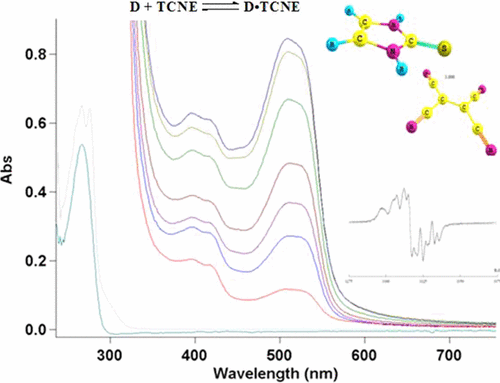
Tetracyanoethylene (TCNE) as one of the most versatile organic compounds is involved in various chemical reactions with electron transfer. Charge transfer complexes (CTCs) of a few antioxidants, nitrogen containing thioamides [pyrrolidine-2-thione (I), 1,3-H-imidazolidine-2-thione (II), 1,3-H-Imidazoline-2-thione (III), pyridine-2-thione (IV), 5-trifluoromethylpyridine-2-thione (V), 4-trifluoromethylpyrimidine-2-thione (VI), quinoline-2-thione (VII), 3,4,5,6-tetrahydropyrimidine-2-thione (VIII)] as π-donors and TCNE as π-acceptor were studied. The DFT PCM/UB3LYP/6-31++G(d,p) and SA-CASSCF quantum chemical calculations were used to study the structures and relative stabilities of these complexes in the ground and lowest excited electronic states. The formation of a weak molecular associates in the chloroform and acetonitrile solutions was confirmed by UV/vis and IR absorption spectroscopy. The stability constants and molar extinction coefficients were estimated by UV/vis spectroscopy. The highest stability in acetonitrile is found for associates formed by quinoline-2-thione and pyridine-2-thione with TCNE, the lowest one is found for CTC formed by imidazolidine-2-thione. Molecular associate formed by pyridine-2-thione and TCNE has the greatest stability in the chloroform solution. 5-Trifluoromethylpyridine-2-thione and 4-trifluoromethylpyrimidine-2-thione do not form CTC in CH3CN due to the presence of an electron acceptor group in the molecules. The molar extinction values of CTC vary within the range of 0.4 × 103 to 1.0 × 104 M–1 cm–1. An analytical strategy of thioamides identification based on wavelength and intensity of CTCs absorption band has been suggested.
中文翻译:

杂环硫代酰胺和四氰基乙烯形成的电荷转移配合物:实验和理论研究
季戊基乙烯(TCNE)作为用途最广泛的有机化合物之一,参与了各种与电子转移有关的化学反应。几种抗氧化剂的电荷转移络合物(CTC),含氮的硫代酰胺[吡咯烷-2-硫酮(I),1,3- H-咪唑烷-2-硫酮(II),1,3- H-咪唑啉-2-硫酮(III),吡啶-2-硫酮(IV),5-三氟甲基吡啶-2-硫酮(V),4-三氟甲基嘧啶-2-硫酮(VI),喹啉-2-硫酮(VII),3、4、5, 6-四氢嘧啶-2-硫酮(VIII)]作为π供体,研究TCNE作为π受体。使用DFT PCM / UB3LYP / 6-31 ++ G(d,p)和SA-CASSCF量子化学计算来研究这些配合物在基态和最低激发电子态下的结构和相对稳定性。通过UV / vis和IR吸收光谱法确认了氯仿和乙腈溶液中弱分子缔合体的形成。通过紫外/可见光谱法估计稳定性常数和摩尔消光系数。喹啉-2-硫酮和吡啶-2-硫酮与TCNE形成的缔合物在乙腈中的稳定性最高,而咪唑烷-2-硫酮形成的CTC的稳定性最低。由吡啶-2-硫酮和TCNE形成的分子缔合物在氯仿溶液中具有最大的稳定性。3 CN由于分子中存在电子受体基团。CTC的摩尔消光值在0.4×10 3至1.0×10 4 M –1 cm –1的范围内变化。提出了基于四氯化碳吸收带的波长和强度的硫酰胺类鉴定的分析策略。
更新日期:2017-09-12
中文翻译:

杂环硫代酰胺和四氰基乙烯形成的电荷转移配合物:实验和理论研究
季戊基乙烯(TCNE)作为用途最广泛的有机化合物之一,参与了各种与电子转移有关的化学反应。几种抗氧化剂的电荷转移络合物(CTC),含氮的硫代酰胺[吡咯烷-2-硫酮(I),1,3- H-咪唑烷-2-硫酮(II),1,3- H-咪唑啉-2-硫酮(III),吡啶-2-硫酮(IV),5-三氟甲基吡啶-2-硫酮(V),4-三氟甲基嘧啶-2-硫酮(VI),喹啉-2-硫酮(VII),3、4、5, 6-四氢嘧啶-2-硫酮(VIII)]作为π供体,研究TCNE作为π受体。使用DFT PCM / UB3LYP / 6-31 ++ G(d,p)和SA-CASSCF量子化学计算来研究这些配合物在基态和最低激发电子态下的结构和相对稳定性。通过UV / vis和IR吸收光谱法确认了氯仿和乙腈溶液中弱分子缔合体的形成。通过紫外/可见光谱法估计稳定性常数和摩尔消光系数。喹啉-2-硫酮和吡啶-2-硫酮与TCNE形成的缔合物在乙腈中的稳定性最高,而咪唑烷-2-硫酮形成的CTC的稳定性最低。由吡啶-2-硫酮和TCNE形成的分子缔合物在氯仿溶液中具有最大的稳定性。3 CN由于分子中存在电子受体基团。CTC的摩尔消光值在0.4×10 3至1.0×10 4 M –1 cm –1的范围内变化。提出了基于四氯化碳吸收带的波长和强度的硫酰胺类鉴定的分析策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号