当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Antibody-conjugated mesoporous silica nanoparticles for brain microvessel endothelial cell targeting
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2017-08-21 00:00:00 , DOI: 10.1039/c7tb01385j Meryem Bouchoucha 1, 2, 3, 4, 5 , Éric Béliveau 2, 3, 4, 6, 7 , Freddy Kleitz 1, 2, 3, 4, 8 , Frédéric Calon 2, 3, 4, 6, 7 , Marc-André Fortin 2, 3, 4, 5, 9
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2017-08-21 00:00:00 , DOI: 10.1039/c7tb01385j Meryem Bouchoucha 1, 2, 3, 4, 5 , Éric Béliveau 2, 3, 4, 6, 7 , Freddy Kleitz 1, 2, 3, 4, 8 , Frédéric Calon 2, 3, 4, 6, 7 , Marc-André Fortin 2, 3, 4, 5, 9
Affiliation
|
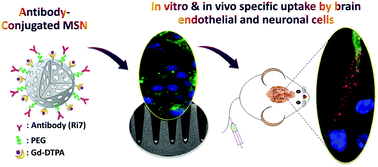
Brain microvessel endothelial cells (BMECs) are the main structural and dynamic components of the blood–brain barrier (BBB), preventing the majority of drugs from reaching the brain. Since BMECs are involved in a wide range of central nervous system diseases, the development of nanocarriers that trigger receptor-mediated uptake in these cells has been suggested as a promising approach to an increased drug delivery to the brain. Here, we report the size and the bioconjugation effects of antibody-conjugated mesoporous silica nanoparticles (MSNs) on in vitro and in vivo targeting ability to BMECs. For this, Ri7 antibody was conjugated to MSNs of two different sizes (50 nm and 160 nm in diameter) through a polyethylene glycol (PEG) linker. The particles were also functionalized with a MRI contrast agent (gadolinium chelate) and with a fluorescent label. The functionalized MSN suspensions showed good colloidal stability. The Ri7 antibody immobilized on the MSN surface maintained its high specific activity and high binding affinity, as demonstrated in vitro. Cells incubated with gadolinium-chelated Ri7-MSNs showed a significant MRI positive contrast enhancement, highlighting the potential of such nanoparticles for theranostic applications. To measure the uptake and affinity of Ri7-MSNs to brain endothelial and neuronal cells, cell uptake studies were performed and a quantitative cellular assay was developed. The results revealed that endocytosis of nanoparticles is mediated by transferrin receptors and that Ri7-MSN cellular uptake is size- and time-dependent. A highest specific uptake was found with 50 nm Ri7-MSNs. Upon intravenous injection, 50 nm Ri7-MSNs were specifically accumulated in BMECs, suggesting the strong potential of antibody-coated nanoparticles for targeting BMECs in vivo. These findings open the door to therapeutic targeting of BMECs, enabling potential therapeutic drug delivery to the brain.
中文翻译:

抗体缀合的介孔二氧化硅纳米粒子用于脑微血管内皮细胞靶向
脑微血管内皮细胞(BMEC)是血脑屏障(BBB)的主要结构和动态组成部分,阻止了大多数药物到达大脑。由于BMEC涉及广泛的中枢神经系统疾病,因此已提出在这些细胞中触发受体介导的摄取的纳米载体的开发是增加向大脑的药物输送的一种有前途的方法。在这里,我们报告抗体和结合的介孔二氧化硅纳米粒子(MSNs)在体外和体内的大小和生物结合作用针对BMEC的定位能力。为此,Ri7抗体通过聚乙二醇(PEG)接头与两种不同大小(直径分别为50 nm和160 nm)的MSN偶联。颗粒还用MRI造影剂(ga螯合物)和荧光标记进行了功能化。功能化的MSN悬浮液显示出良好的胶体稳定性。体外证明,固定在MSN表面的Ri7抗体保持其高比活性和高结合亲和力。用with螯合的Ri7-MSN孵育的细胞显示出显着的MRI阳性对比增强,突出了此类纳米颗粒在治疗学应用中的潜力。为了测量Ri7-MSNs对脑内皮细胞和神经元细胞的摄取和亲和力,进行了细胞摄取研究,并开发了定量细胞测定法。结果表明,纳米颗粒的内吞作用是由转铁蛋白受体介导的,Ri7-MSN细胞的摄取与大小和时间有关。发现50 nm Ri7-MSNs具有最高的比吸收率。静脉注射后,在BMEC中特异积累了50 nm Ri7-MSN,这表明抗体包被的纳米颗粒在体内靶向BMEC的潜力很大。。这些发现为BMEC的治疗靶向性打开了大门,使潜在的治疗性药物可以输送到大脑。
更新日期:2017-09-12
中文翻译:

抗体缀合的介孔二氧化硅纳米粒子用于脑微血管内皮细胞靶向
脑微血管内皮细胞(BMEC)是血脑屏障(BBB)的主要结构和动态组成部分,阻止了大多数药物到达大脑。由于BMEC涉及广泛的中枢神经系统疾病,因此已提出在这些细胞中触发受体介导的摄取的纳米载体的开发是增加向大脑的药物输送的一种有前途的方法。在这里,我们报告抗体和结合的介孔二氧化硅纳米粒子(MSNs)在体外和体内的大小和生物结合作用针对BMEC的定位能力。为此,Ri7抗体通过聚乙二醇(PEG)接头与两种不同大小(直径分别为50 nm和160 nm)的MSN偶联。颗粒还用MRI造影剂(ga螯合物)和荧光标记进行了功能化。功能化的MSN悬浮液显示出良好的胶体稳定性。体外证明,固定在MSN表面的Ri7抗体保持其高比活性和高结合亲和力。用with螯合的Ri7-MSN孵育的细胞显示出显着的MRI阳性对比增强,突出了此类纳米颗粒在治疗学应用中的潜力。为了测量Ri7-MSNs对脑内皮细胞和神经元细胞的摄取和亲和力,进行了细胞摄取研究,并开发了定量细胞测定法。结果表明,纳米颗粒的内吞作用是由转铁蛋白受体介导的,Ri7-MSN细胞的摄取与大小和时间有关。发现50 nm Ri7-MSNs具有最高的比吸收率。静脉注射后,在BMEC中特异积累了50 nm Ri7-MSN,这表明抗体包被的纳米颗粒在体内靶向BMEC的潜力很大。。这些发现为BMEC的治疗靶向性打开了大门,使潜在的治疗性药物可以输送到大脑。


























 京公网安备 11010802027423号
京公网安备 11010802027423号