当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct mapping of the angle-dependent barrier to reaction for Cl + CHD3 using polarized scattering data
Nature Chemistry ( IF 21.8 ) Pub Date : , DOI: 10.1038/nchem.2858 Huilin Pan , Fengyan Wang , Gábor Czakó , Kopin Liu
Nature Chemistry ( IF 21.8 ) Pub Date : , DOI: 10.1038/nchem.2858 Huilin Pan , Fengyan Wang , Gábor Czakó , Kopin Liu
|
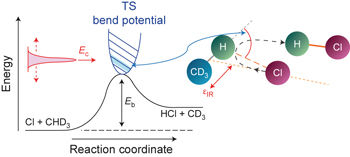
The transition state, which gates and modulates reactive flux, serves as the central concept in our understanding of activated reactions. The barrier height of the transition state can be estimated from the activation energy taken from thermal kinetics data or from the energetic threshold in the measured excitation function (the dependence of reaction cross-sections on initial collision energies). However, another critical and equally important property, the angle-dependent barrier to reaction, has not yet been amenable to experimental determination until now. Here, using the benchmark reaction of Cl + CHD3(v1 = 1) as an example, we show how to map this anisotropic property of the transition state as a function of collision energy from the preferred reactant bond alignment of the backward-scattered products—the imprints of small impact-parameter collisions. The deduced bend potential at the transition state agrees with ab initio calculations. We expect that the method should be applicable to many other direct reactions with a collinear barrier.
中文翻译:

使用极化散射数据直接映射与角度有关的势垒对Cl + CHD 3的反应
门控并调节反应通量的过渡态是我们对活化反应的理解的中心概念。过渡态的势垒高度可以根据从热动力学数据获得的活化能或所测得的激发函数中的高能阈值(反应截面对初始碰撞能的依赖性)来估算。然而,到目前为止,还没有一项重要的,同样重要的特性,即与反应有关的角度依赖性势垒,尚不能通过实验确定。在这里,使用Cl + CHD 3(v 1 = 1)作为示例,我们展示了如何根据向后散射的产物的优选反应物键排列(作为小碰撞参数碰撞的印记),将过渡态的各向异性作为碰撞能量的函数进行映射。推导的过渡态弯曲电位与从头算相符。我们期望该方法应适用于具有共线障碍的许多其他直接反应。
更新日期:2017-09-12
中文翻译:

使用极化散射数据直接映射与角度有关的势垒对Cl + CHD 3的反应
门控并调节反应通量的过渡态是我们对活化反应的理解的中心概念。过渡态的势垒高度可以根据从热动力学数据获得的活化能或所测得的激发函数中的高能阈值(反应截面对初始碰撞能的依赖性)来估算。然而,到目前为止,还没有一项重要的,同样重要的特性,即与反应有关的角度依赖性势垒,尚不能通过实验确定。在这里,使用Cl + CHD 3(v 1 = 1)作为示例,我们展示了如何根据向后散射的产物的优选反应物键排列(作为小碰撞参数碰撞的印记),将过渡态的各向异性作为碰撞能量的函数进行映射。推导的过渡态弯曲电位与从头算相符。我们期望该方法应适用于具有共线障碍的许多其他直接反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号