当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Subcellular Translocation of Protein Disulfide Isomerase on Tetrachlorobenzoquinone-Induced Signaling Shift from Endoplasmic Reticulum Stress to Apoptosis
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.chemrestox.7b00118 Zixuan Liu 1 , Yawen Wang 1 , Yuxin Wang 1 , Wenjing Dong 1 , Xiaomin Xia 1 , Erqun Song 1 , Yang Song 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.chemrestox.7b00118 Zixuan Liu 1 , Yawen Wang 1 , Yuxin Wang 1 , Wenjing Dong 1 , Xiaomin Xia 1 , Erqun Song 1 , Yang Song 1
Affiliation
|
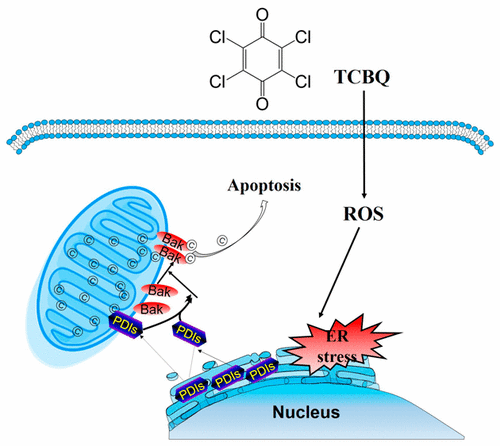
Our previous studies illustrated tetrachlorobenzoquinone (TCBQ)-caused toxicities in neuron-like cells which imply its association with neurodegenerative disorders. Although it is known that TCBQ induces oxidative damage that in turn results in endoplasmic reticulum (ER) stress and apoptosis, it is unclear how TCBQ triggers the signaling switch from pro-survival (to restore cellular homeostasis) to pro-death (trigger apoptosis). Protein disulfide isomerase family proteins (PDIs) regulate the progress of various neurodegenerative disorders, including Parkinson’s disease and Alzheimer’s disease. We tested the hypothesis that subcellular translocation of PDIs implicates the survival/death signaling switch by inducing mitochondrial outer membrane permeabilization (MOMP). The rat pheochromocytoma PC12 cells were exposed to TCBQ, and the concentration-dependent ER stress was observed upon TCBQ treatment, as indicated by the increase in inositol-requiring kinase/endonuclease 1α (IRE1α) phosphorylation, C/EBP homologous protein (CHOP) expression, X-box-binding protein 1 (XBP1) splicing, and caspase 12 activation. Interestingly, pharmacological (or siRNA) abrogation of PDIA1/PDIA3 aggravated the loss of cell viability induced by the relatively low concentration (10 μM) of TCBQ. However, inhibition of PDIA1/PDIA3 rescued the high concentration (20 μM) of TCBQ-induced cell death. Further mechanistic study illustrated that PDIs initially acted to restore cellular homeostasis to pro-survival but that the constant ER stress promoted the signaling switch to pro-apoptotis by the release of PDIA1/PDIA3 from the ER lumen to induce Bak-dependent MOMP. Our findings suggested that subcellular translocation of PDIs determined the “life or death” fate of PC12 cells to TCBQ-induced oxidative insult.
中文翻译:

蛋白二硫键异构酶亚细胞转运对四氯苯醌诱导的内质网应激转凋亡的信号转导的影响
我们以前的研究表明,四氯苯醌(TCBQ)在神经元样细胞中引起的毒性暗示其与神经退行性疾病的关联。尽管已知TCBQ会引起氧化损伤,进而导致内质网(ER)应激和凋亡,但尚不清楚TCBQ如何触发从生存前(恢复细胞稳态)到死亡前(触发凋亡)的信号转换。 。蛋白质二硫键异构酶家族蛋白(PDI)调节各种神经退行性疾病的进展,包括帕金森氏病和阿尔茨海默氏病。我们测试了以下假设,即PDI的亚细胞移位通过诱导线粒体外膜通透性(MOMP)牵涉生存/死亡信号转导。将大鼠嗜铬细胞瘤PC12细胞暴露于TCBQ,TCBQ处理后观察到浓度依赖性的ER应激,这表现为需要肌醇的激酶/核酸内切酶1α(IRE1α)磷酸化,C / EBP同源蛋白(CHOP)表达,X-box结合蛋白1(XBP1)增加。 )剪接,并激活caspase 12。有趣的是,PDIA1 / PDIA3的药理学(或siRNA)废除加剧了由相对较低浓度(10μM)的TCBQ引起的细胞活力丧失。但是,PDIA1 / PDIA3的抑制挽救了高浓度(20μM)的TCBQ诱导的细胞死亡。进一步的机理研究表明,PDI最初起着使细胞稳态恢复至存活的作用,但是恒定的ER应力通过从ER内腔释放PDIA1 / PDIA3来诱导Bak依赖性的MOMP促进了信号转为促凋亡。
更新日期:2017-09-11
中文翻译:

蛋白二硫键异构酶亚细胞转运对四氯苯醌诱导的内质网应激转凋亡的信号转导的影响
我们以前的研究表明,四氯苯醌(TCBQ)在神经元样细胞中引起的毒性暗示其与神经退行性疾病的关联。尽管已知TCBQ会引起氧化损伤,进而导致内质网(ER)应激和凋亡,但尚不清楚TCBQ如何触发从生存前(恢复细胞稳态)到死亡前(触发凋亡)的信号转换。 。蛋白质二硫键异构酶家族蛋白(PDI)调节各种神经退行性疾病的进展,包括帕金森氏病和阿尔茨海默氏病。我们测试了以下假设,即PDI的亚细胞移位通过诱导线粒体外膜通透性(MOMP)牵涉生存/死亡信号转导。将大鼠嗜铬细胞瘤PC12细胞暴露于TCBQ,TCBQ处理后观察到浓度依赖性的ER应激,这表现为需要肌醇的激酶/核酸内切酶1α(IRE1α)磷酸化,C / EBP同源蛋白(CHOP)表达,X-box结合蛋白1(XBP1)增加。 )剪接,并激活caspase 12。有趣的是,PDIA1 / PDIA3的药理学(或siRNA)废除加剧了由相对较低浓度(10μM)的TCBQ引起的细胞活力丧失。但是,PDIA1 / PDIA3的抑制挽救了高浓度(20μM)的TCBQ诱导的细胞死亡。进一步的机理研究表明,PDI最初起着使细胞稳态恢复至存活的作用,但是恒定的ER应力通过从ER内腔释放PDIA1 / PDIA3来诱导Bak依赖性的MOMP促进了信号转为促凋亡。


























 京公网安备 11010802027423号
京公网安备 11010802027423号