当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Size-Dependent Relationships between Protein Stability and Thermal Unfolding Temperature Have Important Implications for Analysis of Protein Energetics and High-Throughput Assays of Protein–Ligand Interactions
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b05684 Matthew D. Watson , Jeremy Monroe , Daniel P. Raleigh 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.jpcb.7b05684 Matthew D. Watson , Jeremy Monroe , Daniel P. Raleigh 1
Affiliation
|
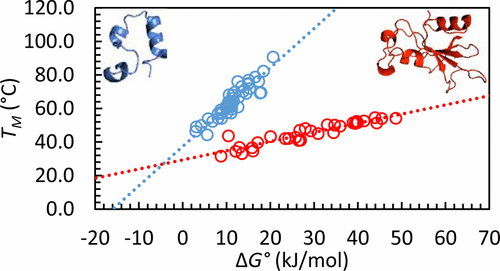
Changes in protein stability are commonly reported as changes in the melting temperature, ΔTM, or as changes in unfolding free energy at a particular temperature, ΔΔG°. Using data for 866 mutants from 16 proteins, we examine the relationship between ΔΔG° and ΔTM. A linear relationship is observed for each protein. The slopes of the plots of ΔTM vs ΔΔG° for different proteins scale as N–1, where N is the number of residues in the protein. Thus, a given change in ΔG° causes a much larger change in TM for a small protein relative to the effect observed for a large protein. The analysis suggests that reasonable estimates of ΔΔG° for a mutant can be obtained by interpolating measured values of TM. The relationship between ΔΔG° and ΔTM has implications for the design and interpretation of high-throughput assays of protein–ligand binding. So-called thermal shift assays rely upon the increase in stability which results from ligand binding to the folded state. Quantitative relationships are derived which show that the observed thermal shift, ΔTM, scales as N–1. Hence, thermal shift assays are considerably less sensitive for ligand binding to larger proteins.
中文翻译:

蛋白质稳定性和热解折叠温度之间的大小相关关系对蛋白质能量学分析和蛋白质-配体相互作用的高通量分析具有重要意义
在蛋白质稳定性的变化通常被报告为在熔融温度变化,Δ Ť中号,或者作为在特定温度下,解折叠ΔΔ自由能变化ģ °。使用数据从16种蛋白质的突变体866,我们检查ΔΔ之间的关系ģ °和Δ Ť中号。对于每种蛋白质观察到线性关系。的Δ的图的斜率Ť中号VSΔΔ ģ °为不同的蛋白质比例为Ñ -1,其中Ñ是在蛋白残基的数目。因此,ΔG °的给定变化会导致ΔG °的更大变化。相对于大蛋白观察到的效果,小蛋白的T M相对。分析表明,ΔΔ的合理估计值G ^ °的突变可以通过内插的测量值来获得Ť中号。ΔΔ之间的关系ģ °和Δ Ť中号具有用于设计和解释影响高通量的测定蛋白-配体结合。所谓的热位移测定法依赖于由于配体结合到折叠状态而引起的稳定性的增加。得出了定量关系,这些关系表明观察到的热位移ΔT M的缩放比例为N –1。因此,热移分析对配体与较大蛋白的结合的敏感性大大降低。
更新日期:2017-09-11
中文翻译:

蛋白质稳定性和热解折叠温度之间的大小相关关系对蛋白质能量学分析和蛋白质-配体相互作用的高通量分析具有重要意义
在蛋白质稳定性的变化通常被报告为在熔融温度变化,Δ Ť中号,或者作为在特定温度下,解折叠ΔΔ自由能变化ģ °。使用数据从16种蛋白质的突变体866,我们检查ΔΔ之间的关系ģ °和Δ Ť中号。对于每种蛋白质观察到线性关系。的Δ的图的斜率Ť中号VSΔΔ ģ °为不同的蛋白质比例为Ñ -1,其中Ñ是在蛋白残基的数目。因此,ΔG °的给定变化会导致ΔG °的更大变化。相对于大蛋白观察到的效果,小蛋白的T M相对。分析表明,ΔΔ的合理估计值G ^ °的突变可以通过内插的测量值来获得Ť中号。ΔΔ之间的关系ģ °和Δ Ť中号具有用于设计和解释影响高通量的测定蛋白-配体结合。所谓的热位移测定法依赖于由于配体结合到折叠状态而引起的稳定性的增加。得出了定量关系,这些关系表明观察到的热位移ΔT M的缩放比例为N –1。因此,热移分析对配体与较大蛋白的结合的敏感性大大降低。



























 京公网安备 11010802027423号
京公网安备 11010802027423号