当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Increase in Solubility of Poorly-Ionizable Pharmaceuticals by Salt Formation: A Case of Agomelatine Sulfonates
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.cgd.7b00805 Eliška Skořepová 1, 2 , Daniel Bím 3 , Michal Hušák 2 , Jiří Klimeš 4 , Argyro Chatziadi 1, 5 , Luděk Ridvan 5 , Tereza Boleslavská 5 , Josef Beránek 5 , Pavel Šebek 5 , Lubomír Rulíšek 3
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1021/acs.cgd.7b00805 Eliška Skořepová 1, 2 , Daniel Bím 3 , Michal Hušák 2 , Jiří Klimeš 4 , Argyro Chatziadi 1, 5 , Luděk Ridvan 5 , Tereza Boleslavská 5 , Josef Beránek 5 , Pavel Šebek 5 , Lubomír Rulíšek 3
Affiliation
|
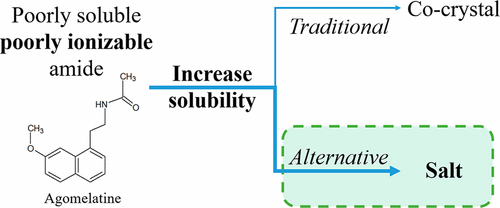
The search for new solid forms of an active pharmaceutical ingredient (API) is an important step in drug development. Often, an API has a low water solubility, which then leads to low oral bioavailability. For basic or acidic APIs, the rational solution is the preparation of salts. For neutral, poorly ionizable, compounds, the cocrystallization is often the only choice. Agomelatine, a poorly soluble “nonionizable” amide acting as a melatonergic antidepressant is a typical representative of such class of compounds. Until recently, the only multicomponent forms of agomelatine were cocrystals. In this work, we report the preparation of three salts of agomelatine (hydrogensulfate, mesylate, and besylate) and their solvated forms, along with their crystallographic characterization. Interestingly, the crystal structures of the solvated and nonsolvated hydrogensulfates were determined from the same crystal via a topotactic transformation. In all of the structures, the agomelatine molecule was positively charged with the amide oxygen being protonated. The salt formation was also confirmed by solid state nuclear magnetic resonance measurements and density functional theory calculations. By sulfonate salt formation, up to ∼200-times faster dissolution of agomelatine was achieved, which proves that salts might be an attractive alternative even for the poorly ionizable compounds.
中文翻译:

通过成盐增加难离子化药物的溶解度:阿戈美拉汀磺酸盐的案例
寻找药物活性成分(API)的新固体形式是药物开发中的重要一步。API通常具有较低的水溶性,从而导致较低的口服生物利用度。对于碱性或酸性API,合理的解决方案是制备盐。对于中性,难离子化的化合物,共结晶通常是唯一的选择。Agomelatine是一种难溶的“非离子化”酰胺,可作为褪黑素抗抑郁药,是这类化合物的典型代表。直到最近,阿戈美拉汀的唯一多组分形式是共晶体。在这项工作中,我们报告了阿戈美拉汀的三种盐(硫酸氢盐,甲磺酸盐和苯磺酸盐)的制备及其溶剂化形式,以及其晶体学表征。有趣的是,溶剂化硫酸盐和非溶剂化硫酸氢盐的晶体结构是通过全能变换从同一晶体确定的。在所有结构中,阿戈美拉汀分子带有被质子化的酰胺氧带正电。固态核磁共振测量和密度泛函理论计算也证实了盐的形成。通过磺酸盐的形成,阿戈美拉汀的溶解速度提高了约200倍,这证明即使对于离子化程度较差的化合物,盐也可能是有吸引力的替代品。固态核磁共振测量和密度泛函理论计算也证实了盐的形成。通过磺酸盐的形成,阿戈美拉汀的溶解速度提高了约200倍,这证明即使对于离子化程度较差的化合物,盐也可能是有吸引力的替代品。固态核磁共振测量和密度泛函理论计算也证实了盐的形成。通过磺酸盐的形成,阿戈美拉汀的溶解速度提高了约200倍,这证明即使对于离子化程度较差的化合物,盐也可能是有吸引力的替代品。
更新日期:2017-09-11
中文翻译:

通过成盐增加难离子化药物的溶解度:阿戈美拉汀磺酸盐的案例
寻找药物活性成分(API)的新固体形式是药物开发中的重要一步。API通常具有较低的水溶性,从而导致较低的口服生物利用度。对于碱性或酸性API,合理的解决方案是制备盐。对于中性,难离子化的化合物,共结晶通常是唯一的选择。Agomelatine是一种难溶的“非离子化”酰胺,可作为褪黑素抗抑郁药,是这类化合物的典型代表。直到最近,阿戈美拉汀的唯一多组分形式是共晶体。在这项工作中,我们报告了阿戈美拉汀的三种盐(硫酸氢盐,甲磺酸盐和苯磺酸盐)的制备及其溶剂化形式,以及其晶体学表征。有趣的是,溶剂化硫酸盐和非溶剂化硫酸氢盐的晶体结构是通过全能变换从同一晶体确定的。在所有结构中,阿戈美拉汀分子带有被质子化的酰胺氧带正电。固态核磁共振测量和密度泛函理论计算也证实了盐的形成。通过磺酸盐的形成,阿戈美拉汀的溶解速度提高了约200倍,这证明即使对于离子化程度较差的化合物,盐也可能是有吸引力的替代品。固态核磁共振测量和密度泛函理论计算也证实了盐的形成。通过磺酸盐的形成,阿戈美拉汀的溶解速度提高了约200倍,这证明即使对于离子化程度较差的化合物,盐也可能是有吸引力的替代品。固态核磁共振测量和密度泛函理论计算也证实了盐的形成。通过磺酸盐的形成,阿戈美拉汀的溶解速度提高了约200倍,这证明即使对于离子化程度较差的化合物,盐也可能是有吸引力的替代品。


























 京公网安备 11010802027423号
京公网安备 11010802027423号