当前位置:
X-MOL 学术
›
Appl. Catal. B Environ. Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Degradation of atrazine by ZnxCu1−xFe2O4 nanomaterial-catalyzed sulfite under UV-visible light irradiation: Green strategy to generate SO4 −
Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2017-09-09 , DOI: 10.1016/j.apcatb.2017.09.001 Ying Huang , Changseok Han , Yiqing Liu , Mallikarjuna N. Nadagouda , Libor Machala , Kevin E. O’Shea , Virender K. Sharma , Dionysios D. Dionysiou
Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2017-09-09 , DOI: 10.1016/j.apcatb.2017.09.001 Ying Huang , Changseok Han , Yiqing Liu , Mallikarjuna N. Nadagouda , Libor Machala , Kevin E. O’Shea , Virender K. Sharma , Dionysios D. Dionysiou
|
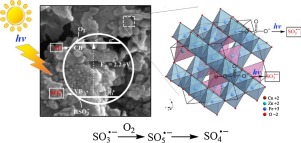
Degradation of atrazine, a widely-used herbicide, by a novel advanced oxidation process was investigated through photo-catalyzing sulfite, the precursor of sulfate radical (SO4−) in this study, by zinc-copper ferrites (ZnxCu1−xFe2O4) under UV-visible light irradiation. The ZnxCu1−xFe2O4 with different ratios of Zn to Cu were synthesized through a facile sol-gel combustion method, and characterized by X-ray powder diffractometry, scanning electron microscopy, transmission electron microscopy, porosimetry, and UV-visible diffuse reflectance spectroscopy, and by a vibrating sample magnetometer and Mössbauer spectrometer. The Zn0.8Cu0.2Fe2O4 demonstrated the highest photocatalytic ability to activate sulfite for the degradation of atrazine. The sulfate radical generated in the UV-visible light/Zn0.8Cu0.2Fe2O4/sulfite system was identified as the main reactive species through radical quenching experiments and measuring two important byproducts (atrazine-desethyl and atrazine-desisopropyl). The XPS spectra of fresh and used catalysts were analyzed to further elucidate the reaction mechanisms. There are two possible approaches to produce SO4 −: the oxidation of sulfite by photo-generated holes and the accelerated decomposition of metal-sulfito complexes (Fe(III)-sulfito and Cu(II)-sulfito) on the surface of Zn0.8Cu0.2Fe2O4. Based on the detected byproducts, the transformation pathways of atrazine by UV–vis light/Zn0.8Cu0.2Fe2O4/sulfite were proposed as well. After the complete decomposition of atrazine, the used catalysts could be magnetically recovered using a magnet and no sulfite remained in the system. The results suggest that the UV-visible light/Zn0.8Cu0.2Fe2O4/sulfite system is a “green” advanced oxidation technology for future application in wastewater treatment.
−: the oxidation of sulfite by photo-generated holes and the accelerated decomposition of metal-sulfito complexes (Fe(III)-sulfito and Cu(II)-sulfito) on the surface of Zn0.8Cu0.2Fe2O4. Based on the detected byproducts, the transformation pathways of atrazine by UV–vis light/Zn0.8Cu0.2Fe2O4/sulfite were proposed as well. After the complete decomposition of atrazine, the used catalysts could be magnetically recovered using a magnet and no sulfite remained in the system. The results suggest that the UV-visible light/Zn0.8Cu0.2Fe2O4/sulfite system is a “green” advanced oxidation technology for future application in wastewater treatment.
中文翻译:

Zn x Cu 1-x Fe 2 O 4纳米材料催化亚硫酸盐在紫外-可见光辐射下降解阿特拉津的绿色策略:生成SO 4-
通过光催化亚硫酸盐(硫酸根自由基(SO 4)的前体),研究了一种新型的高级氧化方法对一种广泛使用的除草剂阿特拉津的降解作用。−)在本研究中,是在紫外可见光照射下使用锌铜铁氧体(Zn x Cu 1-x Fe 2 O 4)制成的。通过简便的溶胶-凝胶燃烧法合成了不同比例的Zn与Cu的Zn x Cu 1-x Fe 2 O 4,并通过X射线粉末衍射,扫描电子显微镜,透射电子显微镜,孔隙率法和紫外光谱进行了表征。可见漫反射光谱,以及振动样品磁力计和Mössbauer光谱仪。Zn 0.8 Cu 0.2 Fe 2 O 4证明了激活亚硫酸盐降解亚硫酸盐的最高光催化能力。在紫外可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4 /亚硫酸盐体系中产生的硫酸根通过自由基猝灭实验和测量两种重要的副产物(阿特拉津-去乙基和阿特拉津-去异丙基)被确定为主要的反应物种。分析了新鲜和使用过的催化剂的XPS光谱,以进一步阐明反应机理。有两种可能的方法可以产生SO 4 -:光生空穴对亚硫酸盐的氧化,并在Zn 0.8 Cu 0.2 Fe 2 O 4的表面上促进金属-亚砜配合物(Fe(III)-亚砜和Cu(II)-亚砜)的分解。基于检测到的副产物,还提出了紫外-可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4 /亚硫酸盐对of去津的转化途径。在r去津完全分解后,可以使用磁铁以磁性方式回收用过的催化剂,并且系统中没有亚硫酸盐残留。结果表明,紫外可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4/亚硫酸盐系统是一种“绿色”先进的氧化技术,可用于未来的废水处理。
-:光生空穴对亚硫酸盐的氧化,并在Zn 0.8 Cu 0.2 Fe 2 O 4的表面上促进金属-亚砜配合物(Fe(III)-亚砜和Cu(II)-亚砜)的分解。基于检测到的副产物,还提出了紫外-可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4 /亚硫酸盐对of去津的转化途径。在r去津完全分解后,可以使用磁铁以磁性方式回收用过的催化剂,并且系统中没有亚硫酸盐残留。结果表明,紫外可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4/亚硫酸盐系统是一种“绿色”先进的氧化技术,可用于未来的废水处理。
更新日期:2017-09-10
 −: the oxidation of sulfite by photo-generated holes and the accelerated decomposition of metal-sulfito complexes (Fe(III)-sulfito and Cu(II)-sulfito) on the surface of Zn0.8Cu0.2Fe2O4. Based on the detected byproducts, the transformation pathways of atrazine by UV–vis light/Zn0.8Cu0.2Fe2O4/sulfite were proposed as well. After the complete decomposition of atrazine, the used catalysts could be magnetically recovered using a magnet and no sulfite remained in the system. The results suggest that the UV-visible light/Zn0.8Cu0.2Fe2O4/sulfite system is a “green” advanced oxidation technology for future application in wastewater treatment.
−: the oxidation of sulfite by photo-generated holes and the accelerated decomposition of metal-sulfito complexes (Fe(III)-sulfito and Cu(II)-sulfito) on the surface of Zn0.8Cu0.2Fe2O4. Based on the detected byproducts, the transformation pathways of atrazine by UV–vis light/Zn0.8Cu0.2Fe2O4/sulfite were proposed as well. After the complete decomposition of atrazine, the used catalysts could be magnetically recovered using a magnet and no sulfite remained in the system. The results suggest that the UV-visible light/Zn0.8Cu0.2Fe2O4/sulfite system is a “green” advanced oxidation technology for future application in wastewater treatment.
中文翻译:

Zn x Cu 1-x Fe 2 O 4纳米材料催化亚硫酸盐在紫外-可见光辐射下降解阿特拉津的绿色策略:生成SO 4-
通过光催化亚硫酸盐(硫酸根自由基(SO 4)的前体),研究了一种新型的高级氧化方法对一种广泛使用的除草剂阿特拉津的降解作用。−)在本研究中,是在紫外可见光照射下使用锌铜铁氧体(Zn x Cu 1-x Fe 2 O 4)制成的。通过简便的溶胶-凝胶燃烧法合成了不同比例的Zn与Cu的Zn x Cu 1-x Fe 2 O 4,并通过X射线粉末衍射,扫描电子显微镜,透射电子显微镜,孔隙率法和紫外光谱进行了表征。可见漫反射光谱,以及振动样品磁力计和Mössbauer光谱仪。Zn 0.8 Cu 0.2 Fe 2 O 4证明了激活亚硫酸盐降解亚硫酸盐的最高光催化能力。在紫外可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4 /亚硫酸盐体系中产生的硫酸根通过自由基猝灭实验和测量两种重要的副产物(阿特拉津-去乙基和阿特拉津-去异丙基)被确定为主要的反应物种。分析了新鲜和使用过的催化剂的XPS光谱,以进一步阐明反应机理。有两种可能的方法可以产生SO 4
 -:光生空穴对亚硫酸盐的氧化,并在Zn 0.8 Cu 0.2 Fe 2 O 4的表面上促进金属-亚砜配合物(Fe(III)-亚砜和Cu(II)-亚砜)的分解。基于检测到的副产物,还提出了紫外-可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4 /亚硫酸盐对of去津的转化途径。在r去津完全分解后,可以使用磁铁以磁性方式回收用过的催化剂,并且系统中没有亚硫酸盐残留。结果表明,紫外可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4/亚硫酸盐系统是一种“绿色”先进的氧化技术,可用于未来的废水处理。
-:光生空穴对亚硫酸盐的氧化,并在Zn 0.8 Cu 0.2 Fe 2 O 4的表面上促进金属-亚砜配合物(Fe(III)-亚砜和Cu(II)-亚砜)的分解。基于检测到的副产物,还提出了紫外-可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4 /亚硫酸盐对of去津的转化途径。在r去津完全分解后,可以使用磁铁以磁性方式回收用过的催化剂,并且系统中没有亚硫酸盐残留。结果表明,紫外可见光/ Zn 0.8 Cu 0.2 Fe 2 O 4/亚硫酸盐系统是一种“绿色”先进的氧化技术,可用于未来的废水处理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号