Tetrahedron ( IF 2.1 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.tet.2017.09.007 Binfang Yuan , Rongxing He , Wei Shen , Ming Li
|
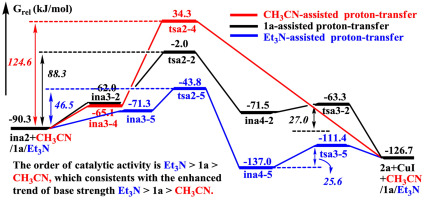
Our calculations find that the base strength is the primary factor that controls the catalytic capability of cocatalysts CH3CN, Propargylic acetates (1a) and Et3N. In the proton-transfer process, the trend of catalytic activity increases in the order: Et3N > 1a > CH3CN, which matches well with the enhanced trend of base strength (Et3N > 1a > CH3CN). Et3N is the most appropriate for the present Cu-catalyzed cycloisomerization of propargylic acetates, which is in agreement with the experimental phenomena. Besides, our calculations give a reasonable explanation for the effects of terminal substituents (H- vs. Ph-) of alkyne on the catalytic reaction.
中文翻译:

碱强度对炔丙基乙酸酯在铜催化的环异构化反应中形成吲哚嗪的影响:DFT研究
我们的计算发现,基本强度是控制助催化剂CH 3 CN,乙酸丙二醇酯(1a)和Et 3 N催化能力的主要因素。在质子转移过程中,催化活性的趋势按以下顺序增加:Et 3 N> 1a > CH 3 CN,这与碱强度的增强趋势非常吻合(Et 3 N> 1a > CH 3 CN)。等3对于当前的炔丙基乙酸酯的Cu催化的环异构化,N是最合适的,这与实验现象一致。此外,我们的计算为炔烃的末端取代基(H-与Ph-)对催化反应的影响提供了合理的解释。


























 京公网安备 11010802027423号
京公网安备 11010802027423号