当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective total synthesis of sacrolide A
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.tetlet.2017.09.017 Tomoyo Mohri , Yusuke Ogura , Ryo Towada , Shigefumi Kuwahara
中文翻译:

cro糖内酯A的对映选择性全合成
更新日期:2017-09-08
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.tetlet.2017.09.017 Tomoyo Mohri , Yusuke Ogura , Ryo Towada , Shigefumi Kuwahara
|
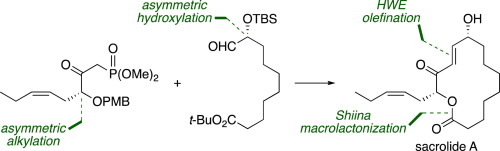
An enantioselective total synthesis of sacrolide A, an antimicrobial and cytotoxic fourteen-membered macrolactonic oxylipin isolated from an edible freshwater cyanobacterium, has been accomplished from a known carboxylic acid in 20% overall yield by a concise ten-step sequence. The key transformations include chiral oxazolidinone-based diastereoselective installation of two hydroxy-bearing stereocenters, the Horner–Wadsworth–Emmons olefination to construct the full carbon skeleton, and the Shiina macrolactonization to establish the fourteen-membered macrolide structure.
中文翻译:

cro糖内酯A的对映选择性全合成
从食用羧酸蓝藻中分离得到的抗菌剂和具有细胞毒性的十四元大环内脂酰氧杂环丁烯醛对映体的全选择性合成已通过一种简明的十步法从已知羧酸中以20%的总收率完成。关键的转变包括基于手性恶唑烷酮的非对映选择性安装的两个带有羟基的立体中心,霍纳-沃兹沃思-埃蒙斯烯化反应以构建完整的碳骨架,以及什叶纳大内酯化以建立十四元大环内酯结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号