当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Addition–Elimination Mechanism of EPSP Synthase through Computer Modeling
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpcb.7b05063 Alberto M. dos Santos 1 , Anderson H. Lima 1 , Cláudio Nahum Alves 2 , Jerônimo Lameira 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.jpcb.7b05063 Alberto M. dos Santos 1 , Anderson H. Lima 1 , Cláudio Nahum Alves 2 , Jerônimo Lameira 1
Affiliation
|
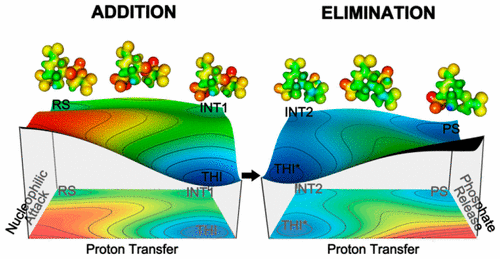
Enolpyruvyl transfer from phosphoenolpyruvate (PEP) to the hydroxyl group of shikimate-5-OH-3-phosphate (S3P) is catalyzed by 5-enolpyruvylshikimate 3-phosphate (EPSP) synthase in a reaction that involves breaking the C–O bond of PEP. Catalysis involves an addition–elimination mechanism with the formation of a tetrahedral intermediate (THI). Experiments have elucidated the mechanism of THI formation and breakdown. However, the catalytic action of EPSP synthase and the individual roles of catalytic residues Asp313 and Glu341 remains unclear. We have used a hybrid quantum mechanical/molecular mechanical (QM/MM) approach to explore the free energy surface in a reaction catalyzed by EPSP synthase. The Glu341 was the most favorable acid/base catalyst. Our results indicate that the protonation of PEP C3 precedes the nucleophilic attack on PEP C2 in the addition mechanism. Also, the breaking of the C–O bond of THI to form an EPSP cation intermediate must occur before proton transfer from PEP C3 to Glu341 in the elimination mechanism. Analysis of the FES supports cationic intermediate formation during the reaction catalyzed by EPSP synthase. Finally, the computational model indicates a proton transfer shift (Hammond shift) from Glu341 to C3 for an enzyme-based reaction with the shifted transition state, earlier than in the reference reaction in water.
中文翻译:

通过计算机建模揭示EPSP合酶的加除机制
5-烯醇丙酮酸shi草酸酯3-磷酸酯(EPSP)合酶催化烯醇丙酮酸从磷酸烯醇丙酮酸酯(PEP)转移至sh草酸-5-OH-3-磷酸酯(S3P)的羟基,该反应涉及破坏PEP的C–O键。催化涉及加成-消除机制,形成四面体中间体(THI)。实验阐明了THI形成和分解的机理。然而,EPSP合酶的催化作用以及催化残基Asp313和Glu341的个别作用仍不清楚。我们已使用混合量子力学/分子力学(QM / MM)方法来探索由EPSP合酶催化的反应中的自由能表面。Glu341是最有利的酸/碱催化剂。我们的结果表明,在加成机理中,PEP C3的质子化先于对PEP C2的亲核攻击。同样,在消除机理中,质子从PEP C3转移到Glu341之前,必须发生THI的C-O键断裂以形成EPSP阳离子中间体的情况。FES的分析支持EPSP合酶催化的反应过程中阳离子中间体的形成。最后,计算模型表明质子从Glu341到C3的质子转移位移(Hammond位移)比在水中的参考反应更早,具有转移状态的基于酶的反应。FES的分析支持EPSP合酶催化的反应过程中阳离子中间体的形成。最后,计算模型表明质子从Glu341到C3的质子转移位移(汉蒙德位移)比在水中的参考反应更早,具有转移状态的基于酶的反应。FES的分析支持EPSP合酶催化的反应过程中阳离子中间体的形成。最后,计算模型表明质子从Glu341到C3的质子转移位移(Hammond位移)比在水中的参考反应更早,具有转移状态的基于酶的反应。
更新日期:2017-09-08
中文翻译:

通过计算机建模揭示EPSP合酶的加除机制
5-烯醇丙酮酸shi草酸酯3-磷酸酯(EPSP)合酶催化烯醇丙酮酸从磷酸烯醇丙酮酸酯(PEP)转移至sh草酸-5-OH-3-磷酸酯(S3P)的羟基,该反应涉及破坏PEP的C–O键。催化涉及加成-消除机制,形成四面体中间体(THI)。实验阐明了THI形成和分解的机理。然而,EPSP合酶的催化作用以及催化残基Asp313和Glu341的个别作用仍不清楚。我们已使用混合量子力学/分子力学(QM / MM)方法来探索由EPSP合酶催化的反应中的自由能表面。Glu341是最有利的酸/碱催化剂。我们的结果表明,在加成机理中,PEP C3的质子化先于对PEP C2的亲核攻击。同样,在消除机理中,质子从PEP C3转移到Glu341之前,必须发生THI的C-O键断裂以形成EPSP阳离子中间体的情况。FES的分析支持EPSP合酶催化的反应过程中阳离子中间体的形成。最后,计算模型表明质子从Glu341到C3的质子转移位移(Hammond位移)比在水中的参考反应更早,具有转移状态的基于酶的反应。FES的分析支持EPSP合酶催化的反应过程中阳离子中间体的形成。最后,计算模型表明质子从Glu341到C3的质子转移位移(汉蒙德位移)比在水中的参考反应更早,具有转移状态的基于酶的反应。FES的分析支持EPSP合酶催化的反应过程中阳离子中间体的形成。最后,计算模型表明质子从Glu341到C3的质子转移位移(Hammond位移)比在水中的参考反应更早,具有转移状态的基于酶的反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号