Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.bmcl.2017.09.017 Bunta Watanabe , Yukiko Tabuchi , Kei Wada , Jun Hiratake
|
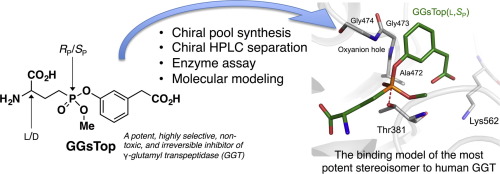
2-Amino-4-{[3-(carboxymethyl)phenoxy](methoxy)phosphoryl}butanoic acid (GGsTop) is a potent, highly selective, nontoxic, and irreversible inhibitor of γ-glutamyl transpeptidase (GGT). GGsTop has been widely used in academic and medicinal research, and also as an active ingredient (Nahlsgen) in commercial anti-aging cosmetics. GGsTop consists of four stereoisomers due to the presence of two stereogenic centers, i.e., the α-carbon atom of the glutamate mimic (l/d) and the phosphorus atom (RP/SP). In this study, each stereoisomer of GGsTop was synthesized stereoselectively and their inhibitory activity against human GGT was evaluated. The l- and d-configurations of each stereoisomer were determined by a combination of a chiral pool synthesis and chiral HPLC analysis. The synthesis of the four stereoisomers of GGsTop used chiral synthetic precursors that were separated by chiral HPLC on a preparative scale. With respect to the configuration of the α-carbon atom of the glutamate mimic, the l-isomer (kon = 174 M−1 s−1) was ca. 8-fold more potent than the d-isomer (kon = 21.5 M−1 s−1). In contrast, the configuration of the phosphorus atom is critical for GGT inhibitory activity. Based on a molecular modeling approach, the absolute configuration of the phosphorus atom of the active GGsTop isomers was postulated to be SP. The SP-isomers inhibited human GGT (kon = 21.5–174 M−1 s−1), while the RP-isomers were inactive even at concentrations of 0.1 mM.
中文翻译:

强效和选择性人γ-谷氨酰转肽酶抑制剂GGs的四种立体异构体的合成和抑制活性的评估
2-氨基-4-{[3-(羧甲基)苯氧基](甲氧基)磷酰基}丁酸(GGsTop)是一种有效,高选择性,无毒且不可逆的γ-谷氨酰转肽酶(GGT)抑制剂。GGsTop已广泛用于学术和医学研究,并且还用作商业抗衰老化妆品中的活性成分(Nahlsgen)。GGsTop由四个立体异构体组成,这是由于存在两个立体生成中心,即谷氨酸模拟物的α-碳原子(l / d)和磷原子(R P / S P)。在这项研究中,立体选择性地合成了GGsTop的每个立体异构体,并评估了它们对人GGT的抑制活性。该升-和d通过手性库合成和手性HPLC分析的组合来确定每种立体异构体的-构型。GGsTop的四种立体异构体的合成使用了手性合成前体,这些前体通过手性HPLC在制备规模上进行了分离。关于谷氨酸模拟物的α-碳原子的构型,l-异构体(k on = 174M -1 s -1)为约。效力比d-异构体高8倍(k on = 21.5 M -1 s -1)。相反,磷原子的构型对于GGT抑制活性至关重要。基于分子建模方法中,活性GGsTop异构体的磷原子的绝对构型,被假定为小号P。该小号P -异构体抑制人GGT(ķ上 = 21.5-174中号-1 小号-1),而[R P -异构体无活性,即使在0.1 mM的浓度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号