Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.bmcl.2017.09.016 Hao Yang , Yifan Ouyang , Hao Ma , Hui Cong , Chunlin Zhuang , Wun-Taai Lok , Zhe Wang , Xuanli Zhu , Yutong Sun , Wei Hong , Hao Wang
|
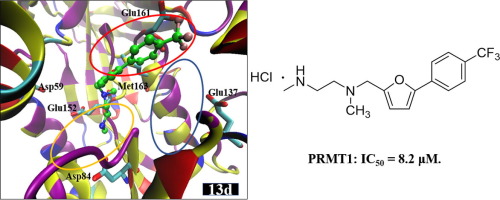
Protein arginine methyltransferase 1 (PRMT1) catalyses the methylation of substrate arginine by transferring the methyl group from SAM (S-adenosyl-l-methionine), which leads to the formation of S-adenosyl homocysteine (SAH) and methylated arginine. We have shown previously that the Asp84 on PRMT1 could be a potential inhibitor binding site. In the current study, 28 compounds were designed and synthesized that were predicted to bind the Asp84 and substrate arginine sites together. Among them, 6 compounds were identified as potential PRMT1 inhibitors, and showed strong inhibitory effects on cancer cell lines, especially HepG2. The most potent PRMT1 inhibitor, compound 13d, was selected for molecular dynamic simulations to investigate binding poses. Based on the free energy calculations and structural analysis, we predicted that the ethylenediamine group would tightly bind to Asp84, and the trifluoromethyl group should occupy part of substrate arginine binding site, which is consistent with our original goal. Our results show for the first time that PRMT1 inhibitors can target the Asp84 binding site, which will be helpful for future drug discovery studies.
中文翻译:

新型PRMT1抑制剂的设计与合成,并通过分子建模研究其结合偏好
蛋白质精氨酸甲基转移酶1(PRMT1)通过从SAM(S-腺苷-1-甲硫氨酸)转移甲基来催化底物精氨酸的甲基化,从而导致S-腺苷高半胱氨酸(SAH)和甲基化的精氨酸的形成。以前我们已经表明PRMT1上的Asp84可能是潜在的抑制剂结合位点。在本研究中,设计和合成了28种化合物,这些化合物预计将Asp84和底物精氨酸位点结合在一起。其中,有6种化合物被鉴定为潜在的PRMT1抑制剂,并且对癌细胞系,尤其是HepG2表现出强烈的抑制作用。最有效的PRMT1抑制剂,化合物13d被选择用于分子动力学模拟以研究结合姿势。基于自由能的计算和结构分析,我们预测乙二胺基团将与Asp84紧密结合,三氟甲基应占据底物精氨酸结合位点的一部分,这与我们最初的目标是一致的。我们的结果首次显示PRMT1抑制剂可以靶向Asp84结合位点,这将有助于未来的药物发现研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号