Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2017-09-08 , DOI: 10.1016/j.apcata.2017.09.008 Linxiao Chen , Joseph P. McCann , Steven L. Tait
|
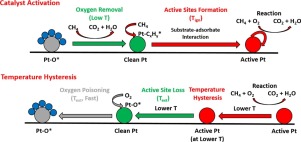
Methane combustion is an industrially important reaction that has been extensively studied. Supported Pt catalysts on Al2O3 exhibit excellent activity in this reaction owing to their CH bond activation capability. Pt/Al2O3 catalyst remains almost inactive until the conversion increases abruptly at the ignition temperature (Tign). The temperature hysteresis behavior has been reported, where after ignition, as the temperature is lowered below Tign, the catalyst continues to exhibit high activity. The activation has been previously attributed to the removal of adsorbed oxygen on Pt sites at Tign, while the hysteresis has been explained by the local temperature elevation caused by the reaction heat. In this work, this behavior was re-examined at a small scale in a fixed-bed flow reactor over a range of pre-treatment and reaction mixture conditions. Low-temperature activation was achieved by pre-treating the catalyst with CH4. Carefully designed control experiments yielded observations that cannot be rationalized by the existing theory, suggesting that it is incomplete. Based on these new results, we propose a more comprehensive theory describing the active sites in the oxidation reaction as methane-derived adsorbed carbon active sites, and their formation as the controlling factor in the catalyst activation. The hysteresis can be better explained by the enhanced stability of active sites under reaction conditions. In situ infrared spectroscopy and mass spectrometry studies were conducted in a flow cell, providing strong evidence supporting the theory. The dissociative adsorption of CH4 was studied under a variety of pre-treatment conditions, and proved to be responsible for the active site formation. The fundamental understanding obtained in this work contributes significant insights to the understanding of reaction mechanism.
中文翻译:

在Pt / Al 2 O 3上甲烷燃烧中催化剂活化和温度滞后的重新检查
甲烷燃烧是工业上重要的反应,已被广泛研究。Al 2 O 3上的担载Pt催化剂由于具有C H键活化能力,因此在该反应中表现出出色的活性。Pt / Al 2 O 3催化剂几乎保持惰性,直到在点火温度(T ign)转化率突然增加为止。已经报道了温度滞后行为,其中在点火之后,当温度降低到低于T ign时,催化剂继续表现出高活性。激活先前已被归因于在在Pt网站去除吸附的氧的牛逼IGN滞后现象是由反应热引起的局部温度升高解释的。在这项工作中,在一系列的预处理和反应混合物条件下,在固定床流动反应器中对这种行为进行了小规模的重新检查。通过用CH 4预处理催化剂可实现低温活化。精心设计的控制实验所产生的观察结果不能被现有理论合理化,这表明它是不完整的。基于这些新结果,我们提出了更全面的理论,将氧化反应中的活性位点描述为甲烷衍生的吸附碳活性位点,并将其形成为催化剂活化的控制因素。滞后现象可以通过反应条件下活性位点稳定性的增强来更好地说明。在流动池中进行了原位红外光谱和质谱研究,为该理论提供了有力的证据。CH 4的解离吸附在各种预处理条件下进行了研究,并证明是造成活性位点形成的原因。在这项工作中获得的基本理解为对反应机理的理解提供了重要的见识。


























 京公网安备 11010802027423号
京公网安备 11010802027423号