Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental and Computational Modeling of H-Bonded Arginine–Tyrosine Groupings in Aprotic Environments
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acsomega.7b00282 Andrew Toyi Banyikwa 1 , Alan Goos 1 , David J Kiemle 2 , Michael A C Foulkes 1 , Mark S Braiman 1
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acsomega.7b00282 Andrew Toyi Banyikwa 1 , Alan Goos 1 , David J Kiemle 2 , Michael A C Foulkes 1 , Mark S Braiman 1
Affiliation
|
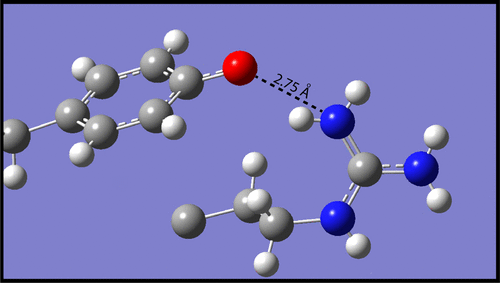
H-bonds between neutral tyrosine and arginine in nonpolar environments are modeled by small-molecule phenol/guanidine complexes. From the temperature and concentration dependence of UV spectra, a value of ΔH° = −74 ± 4 kJ mol–1 is deduced for the formation of H-bonded p-cresol/dodecylguanidine in hexane. ΔE = −71 kJ mol–1 is computed with density functional theory (in vacuo). In dimethyl sulfoxide or crystals, (p-phenolyl)alkylguanidines form head-to-tail homodimers with two strong H-bonding interactions, as evidenced by UV, IR, and NMR spectral shifts, strong IR continuum absorbance bands, and short O···N distances in X-ray crystal structures. Phenol/alkylguanidine H-bonded complexes consist of polarizable rapidly interconverting tautomers, with the proton shift from phenol to guanidine increasing with increase in the polarity of the aprotic solvent. As measured by NMR, both groups in these strongly H-bonded neutral complexes can simultaneously appear to be predominantly protonated. These systems serve as models for the hypothetical hydrogen-Bonded Uncharged (aRginine + tYrosine), or “BU(RY)”, motifs in membrane proteins.
中文翻译:

非质子环境中氢键精氨酸-酪氨酸基团的实验和计算模型
非极性环境中中性酪氨酸和精氨酸之间的氢键由小分子苯酚/胍复合物模拟。根据紫外光谱的温度和浓度依赖性,推导出在己烷中形成氢键对甲酚/十二烷基胍的值 Δ H ° = -74 ± 4 kJ mol –1 。Δ E = -71 kJ mol –1使用密度泛函理论(真空)计算。在二甲基亚砜或晶体中,(对苯酚基)烷基胍形成具有两个强氢键相互作用的头尾同型二聚体,如 UV、IR 和 NMR 光谱位移、强 IR 连续吸收带和短 O·· 所证明的那样·X射线晶体结构中的N距离。苯酚/烷基胍氢键复合物由可极化的快速互变互变异构体组成,随着非质子溶剂极性的增加,质子从苯酚到胍的转移也随之增加。通过 NMR 测量,这些强氢键中性配合物中的两个基团可以同时出现主要质子化。这些系统充当膜蛋白中假设的氢键不带电(精氨酸 + 酪氨酸)或“BU(RY)”基序的模型。
更新日期:2017-09-08
中文翻译:

非质子环境中氢键精氨酸-酪氨酸基团的实验和计算模型
非极性环境中中性酪氨酸和精氨酸之间的氢键由小分子苯酚/胍复合物模拟。根据紫外光谱的温度和浓度依赖性,推导出在己烷中形成氢键对甲酚/十二烷基胍的值 Δ H ° = -74 ± 4 kJ mol –1 。Δ E = -71 kJ mol –1使用密度泛函理论(真空)计算。在二甲基亚砜或晶体中,(对苯酚基)烷基胍形成具有两个强氢键相互作用的头尾同型二聚体,如 UV、IR 和 NMR 光谱位移、强 IR 连续吸收带和短 O·· 所证明的那样·X射线晶体结构中的N距离。苯酚/烷基胍氢键复合物由可极化的快速互变互变异构体组成,随着非质子溶剂极性的增加,质子从苯酚到胍的转移也随之增加。通过 NMR 测量,这些强氢键中性配合物中的两个基团可以同时出现主要质子化。这些系统充当膜蛋白中假设的氢键不带电(精氨酸 + 酪氨酸)或“BU(RY)”基序的模型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号