当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comprehensive Evaluation of the Binding of Lipocalin-Type Prostaglandin D Synthase to Poorly Water-Soluble Drugs
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00590 Yoshiaki Teraoka 1, 2 , Satoshi Kume 1, 3, 4 , Yuxi Lin 3 , Shogo Atsuji 1 , Takashi Inui 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00590 Yoshiaki Teraoka 1, 2 , Satoshi Kume 1, 3, 4 , Yuxi Lin 3 , Shogo Atsuji 1 , Takashi Inui 1
Affiliation
|
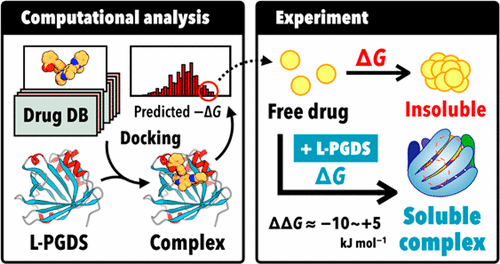
Low water solubility of candidate drug compounds is a major problem in pharmaceutical research and development. We developed a novel drug delivery system (DDS) for poorly water-soluble drugs using lipocalin-type prostaglandin D synthase (L-PGDS), which belongs to the lipocalin superfamily and binds a large variety of hydrophobic molecules. In this study, we comprehensively evaluated the capability of L-PGDS to bind and solubilize various poorly water-soluble drugs using structure-based docking. Docking simulations of 2892 commercially available approved drugs indicated that L-PGDS shows higher binding affinities for various drugs compared with 2-hydroxypropyl-β-cyclodextrin. Five drugs selected from the top 100 with the highest binding affinities for L-PGDS exhibited very low solubility in PBS (pH 7.4). However, in the presence of 1 mM L-PGDS, the apparent solubility of all drugs improved markedly, from 19.5- to 166-fold. Calorimetric experiments on two drugs, telmisartan and imatinib, revealed that L-PGDS forms a 1:2 complex with each drug, with dissociation constants of 0.4–40.0 μM. Kinetic simulations of drug dissolution with L-PGDS indicated that the difference in free energy change (ΔΔG) between the insoluble state and the L-PGDS–bound state are within the range from −10 to +5 kJ mol–1. The ΔΔG value is a critical factor in evaluating whether a poorly water-soluble drug can be solubilized by L-PGDS. Collectively, these results demonstrate that in silico docking is a promising approach for identifying drug molecules suitable for the L-PGDS-based DDS.
中文翻译:

脂联素型前列腺素D合酶与水溶性差的药物的结合的综合评价
候选药物化合物的低水溶性是药物研究和开发中的主要问题。我们使用脂环素型前列腺素D合酶(L-PGDS)开发了一种水溶性差的药物的新型药物递送系统(DDS),该酶属于脂环素超家族并结合了多种疏水分子。在这项研究中,我们使用基于结构的对接综合评估了L-PGDS结合和溶解各种水溶性差的药物的能力。对2892个可商购获得批准的药物的对接模拟表明,与2-羟丙基-β-环糊精相比,L-PGDS对多种药物具有更高的结合亲和力。从前100种药物中,对L-PGDS的结合亲和力最高的五种药物在PBS(pH 7.4)中的溶解度非常低。但是,在存在1 mM L-PGDS的情况下,所有药物的表观溶解度均显着提高,从19.5倍提高到166倍。对两种药物替米沙坦和伊马替尼的量热实验表明,L-PGDS与每种药物形成1:2的络合物,解离常数为0.4–40.0μM。用L-PGDS进行药物溶解的动力学模拟表明,自由能变化的差异(ΔΔ在不溶状态和L-PGDS结合状态之间的G)在-10至+5 kJ mol –1的范围内。的ΔΔ ģ值是在评估是否一个水难溶性药物可以通过L-PGDS被溶解的关键因素。总体而言,这些结果表明,计算机对接技术是用于鉴定适用于基于L-PGDS的DDS的药物分子的有前途的方法。
更新日期:2017-09-08
中文翻译:

脂联素型前列腺素D合酶与水溶性差的药物的结合的综合评价
候选药物化合物的低水溶性是药物研究和开发中的主要问题。我们使用脂环素型前列腺素D合酶(L-PGDS)开发了一种水溶性差的药物的新型药物递送系统(DDS),该酶属于脂环素超家族并结合了多种疏水分子。在这项研究中,我们使用基于结构的对接综合评估了L-PGDS结合和溶解各种水溶性差的药物的能力。对2892个可商购获得批准的药物的对接模拟表明,与2-羟丙基-β-环糊精相比,L-PGDS对多种药物具有更高的结合亲和力。从前100种药物中,对L-PGDS的结合亲和力最高的五种药物在PBS(pH 7.4)中的溶解度非常低。但是,在存在1 mM L-PGDS的情况下,所有药物的表观溶解度均显着提高,从19.5倍提高到166倍。对两种药物替米沙坦和伊马替尼的量热实验表明,L-PGDS与每种药物形成1:2的络合物,解离常数为0.4–40.0μM。用L-PGDS进行药物溶解的动力学模拟表明,自由能变化的差异(ΔΔ在不溶状态和L-PGDS结合状态之间的G)在-10至+5 kJ mol –1的范围内。的ΔΔ ģ值是在评估是否一个水难溶性药物可以通过L-PGDS被溶解的关键因素。总体而言,这些结果表明,计算机对接技术是用于鉴定适用于基于L-PGDS的DDS的药物分子的有前途的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号