当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
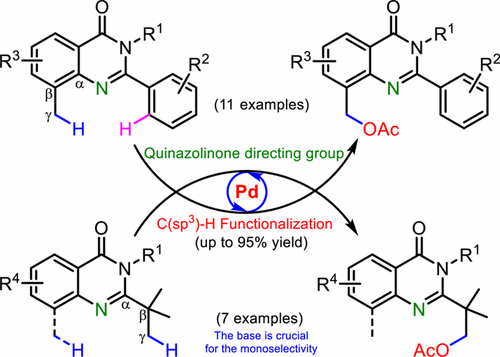
Diversification of Quinazolinones by Pd-Catalyzed C(sp3)-Acetoxylation
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.joc.7b01934 Dnyaneshwar N. Garad 1 , Santosh B. Mhaske 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-09-08 00:00:00 , DOI: 10.1021/acs.joc.7b01934 Dnyaneshwar N. Garad 1 , Santosh B. Mhaske 1
Affiliation
|
The quinazolinone ring has been exploited as a directing group for C(sp3)–H functionalization for the first time. The proximal C−γ(sp3)–H bonds have been oxidized by palladium-catalyzed acetoxylation reaction. Various functional groups on the quinazolinone scaffold were tolerated to provide novel quinazolinone derivatives. The use of base was found to be crucial for the monoselective acetoxylations.
中文翻译:

Pd催化的C(sp 3)-乙酰氧基化使喹唑啉酮类化合物多样化
喹唑啉酮环首次被用作C(sp 3)–H功能化的导向基团。近端的C-γ(sp 3)-H键已通过钯催化的乙酰氧基化反应被氧化。耐受喹唑啉酮支架上的各种官能团以提供新颖的喹唑啉酮衍生物。发现使用碱对于单选择性乙酰氧基化至关重要。
更新日期:2017-09-08
中文翻译:

Pd催化的C(sp 3)-乙酰氧基化使喹唑啉酮类化合物多样化
喹唑啉酮环首次被用作C(sp 3)–H功能化的导向基团。近端的C-γ(sp 3)-H键已通过钯催化的乙酰氧基化反应被氧化。耐受喹唑啉酮支架上的各种官能团以提供新颖的喹唑啉酮衍生物。发现使用碱对于单选择性乙酰氧基化至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号