当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intrinsic Differences in Backbone Dynamics between Wild Type and DNA-Contact Mutants of the p53 DNA Binding Domain Revealed by Nuclear Magnetic Resonance Spectroscopy
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.biochem.7b00514 Juhi A. Rasquinha 1 , Aritra Bej 1 , Shraboni Dutta 1 , Sujoy Mukherjee 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acs.biochem.7b00514 Juhi A. Rasquinha 1 , Aritra Bej 1 , Shraboni Dutta 1 , Sujoy Mukherjee 1
Affiliation
|
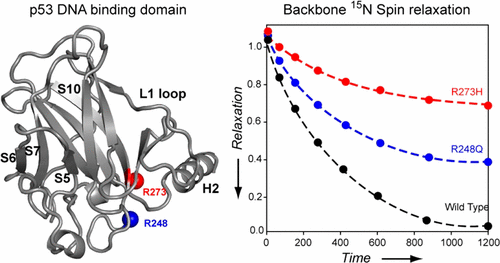
Mutations in p53’s DNA binding domain (p53DBD) are associated with 50% of all cancers, making it an essential system to investigate and understand the genesis and progression of cancer. In this work, we studied the changes in the structure and dynamics of wild type p53DBD in comparison with two of its “hot-spot” DNA-contact mutants, R248Q and R273H, by analysis of backbone amide chemical shift perturbations and 15N spin relaxation measurements. The results of amide chemical shift changes indicated significantly more perturbations in the R273H mutant than in wild type and R248Q p53DBD. Analysis of 15N spin relaxation rates and the resulting nuclear magnetic resonance order parameters suggests that for most parts, the R248Q mutant exhibits limited conformational flexibility and is similar to the wild type protein. In contrast, R273H showed significant backbone dynamics extending up to its β-sandwich scaffold in addition to motions along the DNA binding interface. Furthermore, comparison of rotational correlation times between the mutants suggests that the R273H mutant, with a higher correlation time, forms an enlarged structural fold in comparison to the R248Q mutant and wild type p53DBD. Finally, we identify three regions in these proteins that show conformational flexibility to varying degrees, which suggests that the R273H mutant, in addition to being a DNA-contact mutation, exhibits properties of a conformational mutant.
中文翻译:

核磁共振波谱揭示的野生型和p53 DNA结合域的DNA接触突变体之间的骨干动力学的内在差异。
p53的DNA结合结构域(p53DBD)中的突变与所有癌症的50%相关,这使其成为研究和了解癌症的发生和发展的必不可少的系统。在这项工作中,我们通过分析骨架酰胺化学位移扰动和15 N自旋弛豫,研究了野生型p53DBD与两个“热点” DNA接触突变体R248Q和R273H的结构和动力学变化。测量。酰胺化学位移变化的结果表明,与野生型和R248Q p53DBD相比,R273H突变体的干扰明显更多。分析15N自旋弛豫率和所产生的核磁共振阶数参数表明,对于大多数部分,R248Q突变体表现出有限的构象柔韧性,并且与野生型蛋白相似。相反,除了沿DNA结合界面的运动外,R273H还显示出显着的骨架动力学,一直延伸到其β三明治支架。此外,突变体之间旋转相关时间的比较表明,与R248Q突变体和野生型p53DBD相比,具有较高相关时间的R273H突变体形成了更大的结构折叠。最后,我们在这些蛋白质中鉴定出三个区域,这些区域显示出不同程度的构象柔性,这表明R273H突变体除了是DNA接触突变之外,还表现出构象突变体的特性。
更新日期:2017-09-07
中文翻译:

核磁共振波谱揭示的野生型和p53 DNA结合域的DNA接触突变体之间的骨干动力学的内在差异。
p53的DNA结合结构域(p53DBD)中的突变与所有癌症的50%相关,这使其成为研究和了解癌症的发生和发展的必不可少的系统。在这项工作中,我们通过分析骨架酰胺化学位移扰动和15 N自旋弛豫,研究了野生型p53DBD与两个“热点” DNA接触突变体R248Q和R273H的结构和动力学变化。测量。酰胺化学位移变化的结果表明,与野生型和R248Q p53DBD相比,R273H突变体的干扰明显更多。分析15N自旋弛豫率和所产生的核磁共振阶数参数表明,对于大多数部分,R248Q突变体表现出有限的构象柔韧性,并且与野生型蛋白相似。相反,除了沿DNA结合界面的运动外,R273H还显示出显着的骨架动力学,一直延伸到其β三明治支架。此外,突变体之间旋转相关时间的比较表明,与R248Q突变体和野生型p53DBD相比,具有较高相关时间的R273H突变体形成了更大的结构折叠。最后,我们在这些蛋白质中鉴定出三个区域,这些区域显示出不同程度的构象柔性,这表明R273H突变体除了是DNA接触突变之外,还表现出构象突变体的特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号