当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atomically Dispersed Pt on the Surface of Ni Particles: Synthesis and Catalytic Function in Hydrogen Generation from Aqueous Ammonia–Borane
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acscatal.7b01790 Zhao Li 1, 2 , Teng He 1 , Daiju Matsumura 3 , Shu Miao 1 , Anan Wu 4 , Lin Liu 1 , Guotao Wu 1 , Ping Chen 1, 5
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-09-07 00:00:00 , DOI: 10.1021/acscatal.7b01790 Zhao Li 1, 2 , Teng He 1 , Daiju Matsumura 3 , Shu Miao 1 , Anan Wu 4 , Lin Liu 1 , Guotao Wu 1 , Ping Chen 1, 5
Affiliation
|
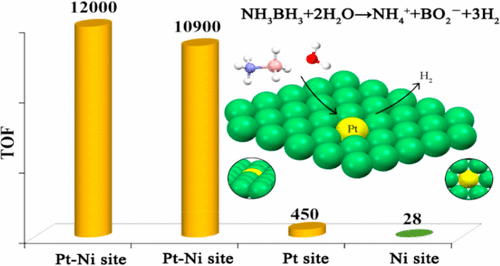
The development of cost-effective and highly efficient catalysts is of scientific importance and practical need in the conversion and utilization of clean energy. One of the strategies fulfilling that demand is to achieve high exposure of a catalytically functional noble metal to reactants to maximize its utilization efficiency. We report herein that the single-atom alloy (SAA) made of atomically dispersed Pt on the surface of Ni particles (Pt is surrounded by Ni atoms) exhibits improved catalytic activity on the hydrolytic dehydrogenation of ammonia–borane, a promising hydrogen storage method for onboard applications. Specifically, an addition of 160 ppm of Pt leads to ca. 3-fold activity improvement in comparison to that of pristine Ni/CNT catalyst. The turnover frequency based on the isolated Pt is 12000 molH2 molPt–1 min–1, which is about 21 times the value of the best Pt-based catalyst ever reported. Our simulation results indicate that the high activity achieved stems from the synergistic effect between Pt and Ni, where the negatively charged Pt (Ptδ-) and positively charged Ni (Niδ+) in the Pt-Ni alloy are prone to interact with H and OH of H2O molecules, respectively, leading to an energetically favorable reaction pathway.
中文翻译:

原子分散在镍颗粒表面的铂:氨-硼烷水溶液制氢的合成及催化功能
在清洁能源的转化和利用中,开发具有成本效益的高效催化剂具有科学重要性和实际需求。满足该需求的策略之一是使催化功能的贵金属高度暴露于反应物以最大化其利用效率。我们在此报告,由原子分散在镍颗粒表面(铂被镍原子包围)的铂构成的单原子合金(SAA)在氨硼烷的水解脱氢方面表现出改善的催化活性,这是一种有前途的储氢方法机载应用程序。具体而言,添加160 ppm的Pt导致大约 与原始Ni / CNT催化剂相比,活性提高了3倍。基于分离的Pt的周转频率为12000 mol H2mol Pt –1 min –1,大约是有史以来最好的Pt基催化剂值的21倍。我们的模拟结果表明,实现高活性的原因在于Pt和Ni之间的协同作用,其中Pt-Ni合金中带负电的Pt(Ptδ-)和带正电的Ni(Niδ+)易于与H相互作用。 H 2 O分子的OH和OH分别导致能量上有利的反应路径。
更新日期:2017-09-07
中文翻译:

原子分散在镍颗粒表面的铂:氨-硼烷水溶液制氢的合成及催化功能
在清洁能源的转化和利用中,开发具有成本效益的高效催化剂具有科学重要性和实际需求。满足该需求的策略之一是使催化功能的贵金属高度暴露于反应物以最大化其利用效率。我们在此报告,由原子分散在镍颗粒表面(铂被镍原子包围)的铂构成的单原子合金(SAA)在氨硼烷的水解脱氢方面表现出改善的催化活性,这是一种有前途的储氢方法机载应用程序。具体而言,添加160 ppm的Pt导致大约 与原始Ni / CNT催化剂相比,活性提高了3倍。基于分离的Pt的周转频率为12000 mol H2mol Pt –1 min –1,大约是有史以来最好的Pt基催化剂值的21倍。我们的模拟结果表明,实现高活性的原因在于Pt和Ni之间的协同作用,其中Pt-Ni合金中带负电的Pt(Ptδ-)和带正电的Ni(Niδ+)易于与H相互作用。 H 2 O分子的OH和OH分别导致能量上有利的反应路径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号