当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetically Trapped Liquid-State Conformers of a Sodiated Model Peptide Observed in the Gas Phase
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.jpca.7b06431 Markus Schneider 1 , Chiara Masellis 2 , Thomas Rizzo 2 , Carsten Baldauf 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.jpca.7b06431 Markus Schneider 1 , Chiara Masellis 2 , Thomas Rizzo 2 , Carsten Baldauf 1
Affiliation
|
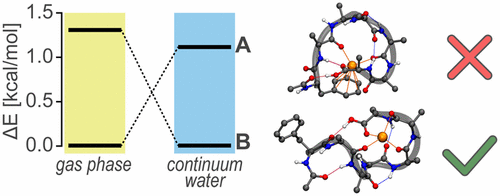
We investigate the peptide AcPheAla5LysH+, a model system for studying helix formation in the gas phase, in order to fully understand the forces that stabilize the helical structure. In particular, we address the question of whether the local fixation of the positive charge at the peptide’s C-terminus is a prerequisite for forming helices by replacing the protonated C-terminal Lys residue by Ala and a sodium cation. The combination of gas-phase vibrational spectroscopy of cryogenically cooled ions with molecular simulations based on density-functional theory (DFT) allows for detailed structure elucidation. For sodiated AcPheAla6, we find globular rather than helical structures, as the mobile positive charge strongly interacts with the peptide backbone and disrupts secondary structure formation. Interestingly, the global minimum structure from simulation is not present in the experiment. We interpret that this is due to high barriers involved in rearranging the peptide–cation interaction that ultimately result in kinetically trapped structures being observed in the experiment.
中文翻译:

气相观察到的固溶模型肽的动力学困住的液态构象异构体
我们研究肽AcPheAla 5 LysH +,该模型系统用于研究气相中的螺旋形成,以充分了解稳定螺旋结构的作用力。特别地,我们解决了以下问题:通过用Ala和钠阳离子取代质子化的C端Lys残基,在肽C端的正电荷的局部固定是否是形成螺旋的先决条件。低温冷却离子的气相振动光谱与基于密度泛函理论(DFT)的分子模拟相结合,可以进行详细的结构阐明。适用于AcPheAla 6,我们发现球形而不是螺旋结构,因为移动的正电荷与肽主链强烈相互作用并破坏二级结构的形成。有趣的是,实验中不存在来自仿真的全局最小结构。我们认为,这是由于重排肽-阳离子相互作用所涉及的高障碍,最终导致在实验中观察到了动力学捕获的结构。
更新日期:2017-09-07
中文翻译:

气相观察到的固溶模型肽的动力学困住的液态构象异构体
我们研究肽AcPheAla 5 LysH +,该模型系统用于研究气相中的螺旋形成,以充分了解稳定螺旋结构的作用力。特别地,我们解决了以下问题:通过用Ala和钠阳离子取代质子化的C端Lys残基,在肽C端的正电荷的局部固定是否是形成螺旋的先决条件。低温冷却离子的气相振动光谱与基于密度泛函理论(DFT)的分子模拟相结合,可以进行详细的结构阐明。适用于AcPheAla 6,我们发现球形而不是螺旋结构,因为移动的正电荷与肽主链强烈相互作用并破坏二级结构的形成。有趣的是,实验中不存在来自仿真的全局最小结构。我们认为,这是由于重排肽-阳离子相互作用所涉及的高障碍,最终导致在实验中观察到了动力学捕获的结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号