当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gas-Phase Reaction of Methyl n-Propyl Ether with OH, NO3, and Cl: Kinetics and Mechanism
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.jpca.7b06877 Jianqiang Zhu 1, 2 , Shuyan Wang 1 , Narcisse T. Tsona 1 , Xiaotong Jiang 1 , Yifeng Wang 3 , Maofa Ge 4 , Lin Du 1, 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acs.jpca.7b06877 Jianqiang Zhu 1, 2 , Shuyan Wang 1 , Narcisse T. Tsona 1 , Xiaotong Jiang 1 , Yifeng Wang 3 , Maofa Ge 4 , Lin Du 1, 2
Affiliation
|
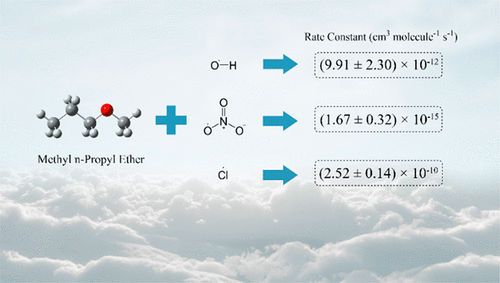
Rate constants at room temperature (293 ± 2 K) and atmospheric pressure for the reaction of methyl n-propyl ether (MnPE), CH3OCH2CH2CH3, with OH and NO3 radicals and the Cl atom have been determined in a 100 L FEP-Teflon reaction chamber in conjunction with gas chromatography-flame ionization detector (GC-FID) as the detection technique. The obtained rate constants k (in units of cm3 molecule–1 s–1) are (9.91 ± 2.30) × 10–12, (1.67 ± 0.32) × 10–15, and (2.52 ± 0.14) × 10–10 for reactions with OH, NO3, and Cl, respectively. The products of these reactions were investigated by gas chromatography-mass spectrometry (GC-MS), and formation mechanisms are proposed for the observed reaction products. Atmospheric lifetimes of the studied ether, calculated from rate constants of the different reactions, reveal that the dominant loss process for MnPE is its reaction with OH, while in coastal areas and in the marine boundary layer, MnPE loss by Cl reaction is also important.
中文翻译:

甲基正丙基醚与OH,NO 3和Cl的气相反应:动力学和机理
甲基正丙基醚(MnPE),CH 3 OCH 2 CH 2 CH 3与OH和NO 3自由基以及Cl原子的反应在室温(293±2 K)和大气压下的速率常数已经确定。 100 L FEP-Teflon反应室与气相色谱-火焰电离检测器(GC-FID)结合作为检测技术。将所得到的速率常数ķ(以cm为单位3分子-1小号-1)为(9.91±2.30)×10 -12,(1.67±0.32)×10 -15,和(2.52±0.14)×10 -10为与OH,NO反应分别为3和Cl。通过气相色谱-质谱法(GC-MS)研究了这些反应的产物,并提出了观察到的反应产物的形成机理。根据不同反应的速率常数计算得出的醚的大气寿命表明,MnPE的主要损失过程是其与OH的反应,而在沿海地区和海洋边界层中,Cl反应引起的MnPE损失也很重要。
更新日期:2017-09-07
中文翻译:

甲基正丙基醚与OH,NO 3和Cl的气相反应:动力学和机理
甲基正丙基醚(MnPE),CH 3 OCH 2 CH 2 CH 3与OH和NO 3自由基以及Cl原子的反应在室温(293±2 K)和大气压下的速率常数已经确定。 100 L FEP-Teflon反应室与气相色谱-火焰电离检测器(GC-FID)结合作为检测技术。将所得到的速率常数ķ(以cm为单位3分子-1小号-1)为(9.91±2.30)×10 -12,(1.67±0.32)×10 -15,和(2.52±0.14)×10 -10为与OH,NO反应分别为3和Cl。通过气相色谱-质谱法(GC-MS)研究了这些反应的产物,并提出了观察到的反应产物的形成机理。根据不同反应的速率常数计算得出的醚的大气寿命表明,MnPE的主要损失过程是其与OH的反应,而在沿海地区和海洋边界层中,Cl反应引起的MnPE损失也很重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号