当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparative Study in Acidic and Alkaline Media of the Effects of pH and Crystallinity on the Hydrogen-Evolution Reaction on MoS2 and MoSe2
ACS Energy Letters ( IF 22.0 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acsenergylett.7b00700 Joshua D. Wiensch 1 , Jimmy John 1 , Jesus M. Velazquez 1 , Daniel A. Torelli 1 , Adam P. Pieterick 1 , Matthew T. McDowell 1 , Ke Sun 1 , Xinghao Zhao 1 , Bruce S. Brunschwig 1 , Nathan S. Lewis 1
ACS Energy Letters ( IF 22.0 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acsenergylett.7b00700 Joshua D. Wiensch 1 , Jimmy John 1 , Jesus M. Velazquez 1 , Daniel A. Torelli 1 , Adam P. Pieterick 1 , Matthew T. McDowell 1 , Ke Sun 1 , Xinghao Zhao 1 , Bruce S. Brunschwig 1 , Nathan S. Lewis 1
Affiliation
|
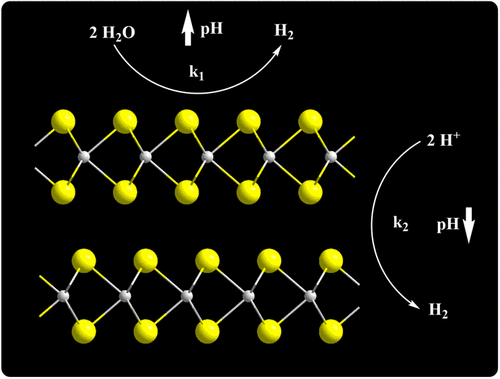
Single crystals of n-type MoS2 and n-MoSe2 showed higher electrocatalytic activity for the evolution of H2(g) in alkaline solutions than in acidic solutions. The overpotentials required to drive hydrogen evolution at −10 mA cm–2 of current density for MoS2 samples were −0.76 ± 0.13 and −1.03 ± 0.21 V when in contact with 1.0 M NaOH(aq) and 1.0 M H2SO4(aq), respectively. For MoSe2 samples, the overpotentials at −10 mA cm–2 were −0.652 ± 0.050 and −0.709 ± 0.073 V in contact with 1.0 M KOH(aq) and 1.0 M H2SO4(aq), respectively. Single crystals from two additional sources were also tested, and the absolute values of the measured overpotentials were consistently less (by 460 ± 250 mV) in alkaline solutions than in acidic solutions. When electrochemical etching was used to create edge sites on the single crystals, the kinetics improved in acid but changed little in alkaline media. The overpotentials measured for polycrystalline thin films (PTFs) and amorphous forms of MoS2 showed less sensitivity to pH and edge density than was observed for single crystals and showed enhanced kinetics in acid when compared to alkaline solutions. These results suggest that the active sites for hydrogen evolution on MoS2 and MoSe2 are different in alkaline and acidic media. Thus, while edges are known to serve as active sites in acidic media, in alkaline media it is more likely that terraces function in this role.
中文翻译:

pH和结晶度对MoS 2和MoSe 2的氢演化反应影响的酸性和碱性介质比较研究
在酸性溶液中,n型MoS 2和n-MoSe 2单晶对H 2(g)的析出表现出更高的电催化活性。在-10毫安厘米需要驱动析氢超电势-2的电流密度为的MoS 2个样品是-0.76±0.13和-1.03±0.21 v当在用1.0M NaOH(水溶液)和1.0 MH接触2 SO 4(水溶液), 分别。对于MoSe 2样品,与1.0 M KOH(aq)和1.0 MH 2 SO 4接触时,−10 mA cm –2处的过电势为-0.652±0.050和-0.709±0.073 V(aq)。还测试了来自其他两个来源的单晶,碱性溶液中测得的超电势绝对值始终比酸性溶液中小(460±250 mV)。当使用电化学蚀刻在单晶上产生边缘位点时,动力学在酸中有所改善,但在碱性介质中几乎没有变化。与单晶相比,多晶薄膜(PTF)和无定形形式的MoS 2的超电势对pH和边缘密度的敏感性更低,并且在酸中的动力学增强。这些结果表明,在MoS 2和MoSe 2上析氢的活性位点在碱性和酸性介质中是不同的。因此,尽管已知边缘在酸性介质中充当活性位点,但在碱性介质中,梯田更有可能发挥这种作用。
更新日期:2017-09-06
中文翻译:

pH和结晶度对MoS 2和MoSe 2的氢演化反应影响的酸性和碱性介质比较研究
在酸性溶液中,n型MoS 2和n-MoSe 2单晶对H 2(g)的析出表现出更高的电催化活性。在-10毫安厘米需要驱动析氢超电势-2的电流密度为的MoS 2个样品是-0.76±0.13和-1.03±0.21 v当在用1.0M NaOH(水溶液)和1.0 MH接触2 SO 4(水溶液), 分别。对于MoSe 2样品,与1.0 M KOH(aq)和1.0 MH 2 SO 4接触时,−10 mA cm –2处的过电势为-0.652±0.050和-0.709±0.073 V(aq)。还测试了来自其他两个来源的单晶,碱性溶液中测得的超电势绝对值始终比酸性溶液中小(460±250 mV)。当使用电化学蚀刻在单晶上产生边缘位点时,动力学在酸中有所改善,但在碱性介质中几乎没有变化。与单晶相比,多晶薄膜(PTF)和无定形形式的MoS 2的超电势对pH和边缘密度的敏感性更低,并且在酸中的动力学增强。这些结果表明,在MoS 2和MoSe 2上析氢的活性位点在碱性和酸性介质中是不同的。因此,尽管已知边缘在酸性介质中充当活性位点,但在碱性介质中,梯田更有可能发挥这种作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号