JAMA Psychiatry ( IF 25.8 ) Pub Date : 2017-09-01 , DOI: 10.1001/jamapsychiatry.2017.1874 Sharon L Walsh 1 , Sandra D Comer 2 , Michelle R Lofwall 1 , Bradley Vince 3 , Naama Levy-Cooperman 4 , Debra Kelsh 3 , Marion A Coe 1 , Jermaine D Jones 2 , Paul A Nuzzo 1 , Fredrik Tiberg 5 , Behshad Sheldon 6 , Sonnie Kim 6
|
Importance Buprenorphine is an efficacious, widely used treatment for opioid use disorder (OUD). Daily oral transmucosal formulations can be associated with misuse, diversion, and nonadherence; these limitations may be obviated by a sustained release formulation.
Objective To evaluate the ability of a novel, weekly, subcutaneous buprenorphine depot formulation, CAM2038, to block euphorigenic opioid effects and suppress opioid withdrawal in non–treatment-seeking individuals with OUD.
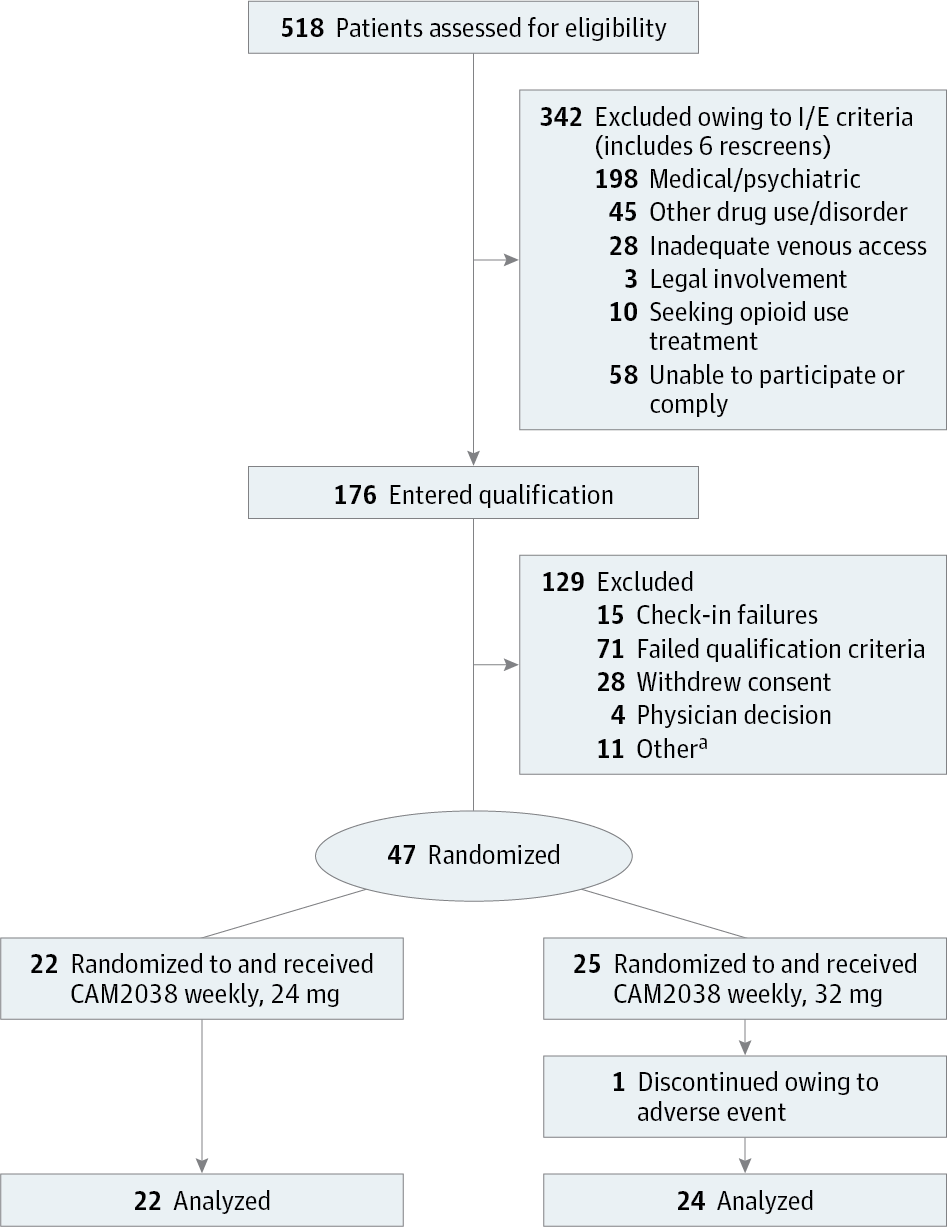
Design, Setting, and Participants This multisite, double-blind, randomized within-patient study was conducted at 3 controlled inpatient research facilities. It involved 47 adults with DSM-V moderate-to-severe OUD. The study was conducted from October 12, 2015 (first patient enrolled), to April 21, 2016 (last patient visit).
Interventions A total of five 3-day test sessions evaluated the response to hydromorphone (0, 6, and 18 mg intramuscular in random order; 1 dose/session/day). After the first 3-day session (ie, qualification phase), participants were randomized to either CAM2038 weekly at 24 mg (n = 22) or 32 mg (n = 25); the assigned CAM2038 dose was given twice, 1 week apart (day 0 and 7). Four sets of sessions were conducted after randomization (days 1-3, 4-6, 8-10, and 11-13).
Main Outcomes and Measures The primary end point was maximum rating on the visual analog scale for drug liking. Secondary end points included other visual analog scale (eg, high and desire to use), opioid withdrawal scales, and physiological and pharmacokinetic outcomes.
Results A total of 46 of 47 randomized participants (mean [SD] age, 35.5 [9] years; 76% male [n = 35]) completed the study. Both weekly CAM2038 doses produced immediate and sustained blockade of hydromorphone effects (liking maximum effect, CAM2038, 24 mg: effect size, 0.813; P < .001, and CAM2038, 32 mg: effect size, 0.753; P < .001) and suppression of withdrawal (Clinical Opiate Withdrawal Scale, CAM2038, 24 mg: effect size, 0.617; P < .001, and CAM2038, 32 mg: effect size, 0.751; P < .001). CAM2038 produces a rapid initial rise of buprenorphine in plasma with maximum concentration around 24 hours, with an apparent half-life of 4 to 5 days and approximately 50% accumulation of trough concentration from first to second dose (trough concentration = 0.822 and 1.23 ng/mL for weeks 1 and 2, respectively, with 24 mg; trough concentration = 0.993 and 1.47 ng/mL for weeks 1 and 2, respectively, with 32 mg).
Conclusions and Relevance CAM2038 weekly, 24 and 32 mg, was safely tolerated and produced immediate and sustained opioid blockade and withdrawal suppression. The results support the use of this depot formulation for treatment initiation and stabilization of patients with OUD, with the further benefit of obviating the risk for misuse and diversion of daily buprenorphine while retaining its therapeutic benefits.
Trial Registration Clinicaltrials.gov Identifier: NCT02611752
中文翻译:

阿片类药物使用障碍个体中丁丙诺啡每周注射液(CAM2038)和氢吗啡酮阻滞的作用:一项随机临床试验。
重要性 丁丙诺啡是一种有效的,广泛使用的阿片类药物使用障碍(OUD)疗法。每日口服透粘膜制剂可能与滥用,转移和不遵守有关;这些限制可以通过缓释制剂来消除。
目的 评估每周一次的新型皮下丁丙诺啡储库制剂CAM2038阻断非寻求治疗的OUD患者的欣快性阿片样物质作用并抑制阿片样物质戒断的能力。
设计,设置和参加者 该多站点,双盲,随机住院患者研究是在3个受控制的住院研究机构进行的。它涉及47位DSM-V成人中度至重度OUD的成年人。该研究于2015年10月12日(首次招募患者)至2016年4月21日(最后一次就诊)进行。
干预 总共进行了5次为期3天的测试,评估了对氢吗啡酮的反应(随机给予0、6和18 mg肌内注射; 1剂量/疗程/天)。在第一个为期3天的会议(即资格评定阶段)之后,将参与者随机分为CAM2038每周一次,剂量为24 mg(n = 22)或32 mg(n = 25)。分配CAM2038剂量两次,间隔1周(第0天和第7天)。随机分组后(第1-3天,4-6天,8-10天和11-13天)进行四组训练。
主要结果和措施 主要终点是在视觉模拟量表上对药物喜欢程度的最高评价。次要终点包括其他视觉模拟量表(例如,较高的使用意愿),阿片类药物戒断量表以及生理和药代动力学结果。
结果 47位随机参与者中的46位(平均[SD]年龄,35.5 [9]岁; 76%的男性[n = 35])完成了研究。每周两次CAM2038剂量均可立即和持续阻断氢吗啡酮的作用(最有效的作用是CAM2038,24 mg:作用量0.813;P <.001,而CAM2038的作用是32 mg:作用量0.753;P <.001),并且抑制戒断的时间(临床阿片戒断量表,CAM2038,24 mg:效应量,0.617; P <.001和CAM2038,32 mg:效应量,0.751; P <.001)。CAM2038在血浆中出现丁丙诺啡的快速初始上升,最大浓度约为24小时,表观半衰期为4至5天,从第一剂到第二剂的谷浓度约有50%累积(谷浓度= 0.822和1.23 ng /第1周和第2周,每毫升分别为24毫克(32毫克);第1周和第2周,低谷浓度分别为0.993和1.47 ng / mL(32毫克)。
结论和相关性 每周安全地耐受CAM2038,分别为24和32 mg,并能产生立即和持续的阿片类药物阻滞和戒断反应。结果支持了这种长效制剂用于OUD患者治疗的开始和稳定,其进一步的好处是消除了日常丁丙诺啡的误用和转移风险,同时又保留了其治疗益处。
试用注册 Clinicaltrials.gov标识符:NCT02611752


























 京公网安备 11010802027423号
京公网安备 11010802027423号