Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spellbinding Effects of the Acidic COOH-Terminus of Factor Va Heavy Chain on Prothrombinase Activity and Function
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acsomega.7b00769 Jamila Hirbawi 1 , Michael Kalafatis 1, 2
ACS Omega ( IF 4.1 ) Pub Date : 2017-09-06 00:00:00 , DOI: 10.1021/acsomega.7b00769 Jamila Hirbawi 1 , Michael Kalafatis 1, 2
Affiliation
|
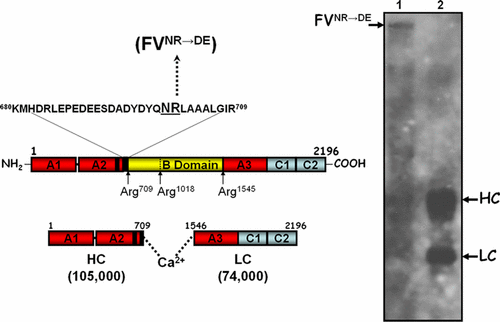
Human factor Va (hfVa) is the important regulatory subunit of prothrombinase. Recent modeling data have suggested a critical role for amino acid Arg701 of hfVa for human prothrombin (hPro) activation by prothrombinase. Furthermore, it has also been demonstrated that hfVa has a different effect than that of bovine fVa on prethrombin-1 activation by prothrombinase. The difference between the two cofactor molecules was also found within the Asn700–Arg701 dipeptide in the human factor V (hfV) molecule, which is replaced by the Asp–Glu sequence in bfV. As a consequence, we produced a recombinant hfV (rhfV) molecule with the substitution 700NR701→DE. rhfVNR→DE together with the wild-type molecule (rhfVWT) were expressed in COS7 cells, purified, and tested for their capability to function within prothrombinase. Kinetic studies showed that the Kd of rhfVaNR→DE for human fXa as well as the kcat and Km of prothrombinase made with rhfVaNR→DE for hPro activation were similar to the values obtained following hPro activation by prothrombinase made with rhfVaWT. Remarkably, sodium dodecyl sulfate polyacrylamide gel electrophoresis analyses of hPro activation time courses demonstrated that the rate of cleavage of hPro by prothrombinase reconstituted with rhfVaNR→DE was significantly delayed with substantial accumulation of meizothrombin, and delayed thrombin generation, when compared to activation of hPro by prothrombinase made with rhfVaWT. These unanticipated results provide significant insights on the role of the carboxyl-terminal end of the heavy chain of hfVa for hPro cleavage and activation by prothrombinase and show that residues 700NR701 regulate at least in part the enzyme–substrate/product interaction during fibrin clot formation.
中文翻译:

因子Va重链的酸性COOH末端对凝血酶原活性和功能的抑制作用
人因子Va(hfVa)是凝血酶原的重要调节亚基。最近的建模数据表明,hfVa的Arg 701氨基酸对凝血酶原酶激活人凝血酶原(hPro)的作用至关重要。此外,还证明了hfVa与牛fVa在凝血酶原酶激活凝血酶原1上具有不同的作用。在人因子V(hfV)分子的Asn 700 –Arg 701二肽中也发现了两个辅助因子分子之间的差异,该差异被bfV中的Asp–Glu序列取代。结果,我们产生了具有替换700 NR 701 →DE的重组hfV(rhfV)分子。rhfV NR→DE与野生型分子(rhfV WT)一起在COS7细胞中表达,纯化并测试了它们在凝血酶原中的功能。动力学研究表明,人fXa的rhfVa NR→DE的K d以及用于hPro激活的rhfVa NR→DE制备的凝血酶原酶的k cat和K m与通过rhfVa WT的凝血酶原激活hPro所获得的值相似。。值得注意的是,hPro活化时间过程的十二烷基硫酸钠聚丙烯酰胺凝胶电泳分析表明,用rhfVa NR→DE重构的凝血酶原酶裂解hPro的速率与用rhfVa WT制成的凝血酶原酶激活hPro相比,Meizothrombin的大量积累显着延迟了凝血酶的生成,并延迟了凝血酶的生成。这些出乎意料的结果提供了关于hfVa重链羧基末端对凝血酶原酶裂解和激活hPro的作用的重要见解,并表明残基700 NR 701至少部分调节了纤维蛋白凝块中酶-底物/产物的相互作用。编队。
更新日期:2017-09-06
中文翻译:

因子Va重链的酸性COOH末端对凝血酶原活性和功能的抑制作用
人因子Va(hfVa)是凝血酶原的重要调节亚基。最近的建模数据表明,hfVa的Arg 701氨基酸对凝血酶原酶激活人凝血酶原(hPro)的作用至关重要。此外,还证明了hfVa与牛fVa在凝血酶原酶激活凝血酶原1上具有不同的作用。在人因子V(hfV)分子的Asn 700 –Arg 701二肽中也发现了两个辅助因子分子之间的差异,该差异被bfV中的Asp–Glu序列取代。结果,我们产生了具有替换700 NR 701 →DE的重组hfV(rhfV)分子。rhfV NR→DE与野生型分子(rhfV WT)一起在COS7细胞中表达,纯化并测试了它们在凝血酶原中的功能。动力学研究表明,人fXa的rhfVa NR→DE的K d以及用于hPro激活的rhfVa NR→DE制备的凝血酶原酶的k cat和K m与通过rhfVa WT的凝血酶原激活hPro所获得的值相似。。值得注意的是,hPro活化时间过程的十二烷基硫酸钠聚丙烯酰胺凝胶电泳分析表明,用rhfVa NR→DE重构的凝血酶原酶裂解hPro的速率与用rhfVa WT制成的凝血酶原酶激活hPro相比,Meizothrombin的大量积累显着延迟了凝血酶的生成,并延迟了凝血酶的生成。这些出乎意料的结果提供了关于hfVa重链羧基末端对凝血酶原酶裂解和激活hPro的作用的重要见解,并表明残基700 NR 701至少部分调节了纤维蛋白凝块中酶-底物/产物的相互作用。编队。



























 京公网安备 11010802027423号
京公网安备 11010802027423号