当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystalline nickel manganese antimonate as a stable water-oxidation catalyst in aqueous 1.0 M H2SO4
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2017-08-10 00:00:00 , DOI: 10.1039/c7ee01486d Ivan A. Moreno-Hernandez 1, 2, 3, 4 , Clara A. MacFarland 1, 2, 3, 4 , Carlos G. Read 1, 2, 3, 4 , Kimberly M. Papadantonakis 1, 2, 3, 4 , Bruce S. Brunschwig 2, 3, 4, 5 , Nathan S. Lewis 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2017-08-10 00:00:00 , DOI: 10.1039/c7ee01486d Ivan A. Moreno-Hernandez 1, 2, 3, 4 , Clara A. MacFarland 1, 2, 3, 4 , Carlos G. Read 1, 2, 3, 4 , Kimberly M. Papadantonakis 1, 2, 3, 4 , Bruce S. Brunschwig 2, 3, 4, 5 , Nathan S. Lewis 1, 2, 3, 4, 5
Affiliation
|
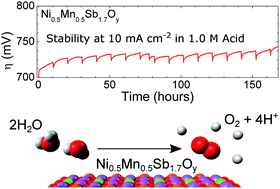
Water oxidation is a required half-reaction for electrochemical water splitting. To date, the only well-established active oxygen-evolution catalysts stable under operating conditions and at rest in acidic aqueous media contain Ru or Ir, two of the scarcest non-radioactive elements on Earth. We report herein a nickel-manganese antimonate electrocatalyst with a rutile-type crystal structure that requires an initial voltammetric overpotential of 672 ± 9 mV to catalyze the oxidation of water to O2(g) at a rate corresponding to 10 mA cm−2 of current density when operated in contact with 1.0 M sulfuric acid. Under galvanostatic control, the overpotential initially rose from 670 mV but was then stable at 735 ± 10 mV for 168 h of continuous operation at 10 mA cm−2. We additionally provide an in-depth evaluation of the stability of the nickel-manganese antimonate electrocatalyst, including elemental characterization of the surface, bulk, and electrolyte before and after electrochemical operation.
中文翻译:

结晶的锑酸锰镍锰酸盐在1.0 MH 2 SO 4水溶液中作为稳定的水氧化催化剂
水氧化是电化学水分解所需的半反应。迄今为止,唯一公认的在运行条件下和在酸性水性介质中静止时稳定的活性氧释放催化剂都含有Ru或Ir,这是地球上最稀缺的两种非放射性元素。我们在此报告了一种具有金红石型晶体结构的镍锰锑酸电催化剂,其初始伏安电位为672±9 mV,才能以相当于10 mA cm -2的速率催化水氧化为O 2(g)。与1.0 M硫酸接触时的最大电流密度。在恒电流控制下,过电势最初从670 mV上升,但随后在10 mA cm -2的连续操作时间168 h中稳定在735±10 mV。。我们还提供了镍锰锑酸镍电催化剂稳定性的深入评估,包括电化学操作前后的表面,块体和电解质的元素表征。
更新日期:2017-09-06
中文翻译:

结晶的锑酸锰镍锰酸盐在1.0 MH 2 SO 4水溶液中作为稳定的水氧化催化剂
水氧化是电化学水分解所需的半反应。迄今为止,唯一公认的在运行条件下和在酸性水性介质中静止时稳定的活性氧释放催化剂都含有Ru或Ir,这是地球上最稀缺的两种非放射性元素。我们在此报告了一种具有金红石型晶体结构的镍锰锑酸电催化剂,其初始伏安电位为672±9 mV,才能以相当于10 mA cm -2的速率催化水氧化为O 2(g)。与1.0 M硫酸接触时的最大电流密度。在恒电流控制下,过电势最初从670 mV上升,但随后在10 mA cm -2的连续操作时间168 h中稳定在735±10 mV。。我们还提供了镍锰锑酸镍电催化剂稳定性的深入评估,包括电化学操作前后的表面,块体和电解质的元素表征。


























 京公网安备 11010802027423号
京公网安备 11010802027423号